EPA revokes scientific finding that underpinned US fight against climate change
On Feb. 12 2026, the Trump administration revoked a scientific finding that long has been the central basis…

On Feb. 12 2026, the Trump administration revoked a scientific finding that long has been the central basis…
On Feb. 10, 2026, The South Carolina Department of Public Health (DPH) is reporting 13 new cases of…

On Feb. 10, 2026, an Annenberg survey finds that a sizable majority of Americans think the three vaccines…

On Feb. 10, 2026, National Institutes of Health (NIH) researchers announced they have developed a digital replica of…

On Feb. 10, 2026, France announced late last week that it would be awarding funds to 46 scientists…

On Feb. 9, 2026, Eli Lilly and Orna Therapeutics, a biotechnology company dedicated to engineering immune cells in…

On Feb. 9, 2026, a study from Centers for Disease Control and Prevention (CDC) finds Candida auris is…

On Feb. 9, 2026, researchers at Penn State reported that amino acids, the building blocks necessary for life,…

On Feb. 7, 2026, Genentech, a member of the Roche Group, announced late-breaking data from the Phase III…
On Feb. 6, 2026, A study by federal and state health researchers indicates invasive Escherichia coli infections are…

On Feb. 4, 2026, the U.S. Centers for Disease Control and Prevention (CDC) reported the 2024-25 COVID-19 vaccine was…
On Feb. 4, 2026, Keck Medicine of the University of Southern California (USC) announced it is conducting an early phase clinical…
On Feb. 3, 2026, the World Health Organization (WHO) and its International Agency for Research on Cancer (IARC)…

On Feb. 3, 2026, Governor Pritzker announced the State of Illinois is joining the World Health Organization’s (WHO)…
On Feb. 2, 2026, two detainees at the nation’s only current immigrant family detention center, 70 miles south…

On Feb. 2, 2026, University of Utah scientists reports an analysis of hair samples shows precipitous reductions in…

On Jan. 31, 2026, a research team from the Institute of Science Tokyo reported they became the first…
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…

On Jan. 30, 2026, the U.S. Centers for Disease Control and Prevention (CDC) confirmed 172 new measles cases…

On Jan. 29, 2026, in a new study from Sweden, researchers found no statistically significant difference in rates…

On Jan. 28, 2026, U.S. drugmaker Eli Lilly announces signing an agreement worth up to $1.12 billion with…
On Jan. 28, 2026, the U.S. Environmental Protection Agency (EPA) launched the first step of its expedited review…

On Jan. 28, 2026, researchers at Google DeepMind have unveiled their latest artificial intelligence tool and claimed it…
On Jan. 27, 2026, a University of Cambridge study shows menopause is linked to reductions in grey matter…

On Jan. 27, 2026, South Carolina reported a surge to 789 measles cases, state health data showed, overtaking…

On Jan. 26, 2026, the American Academy of Pediatrics (AAP) released its 2026 immunization schedule and continues to…
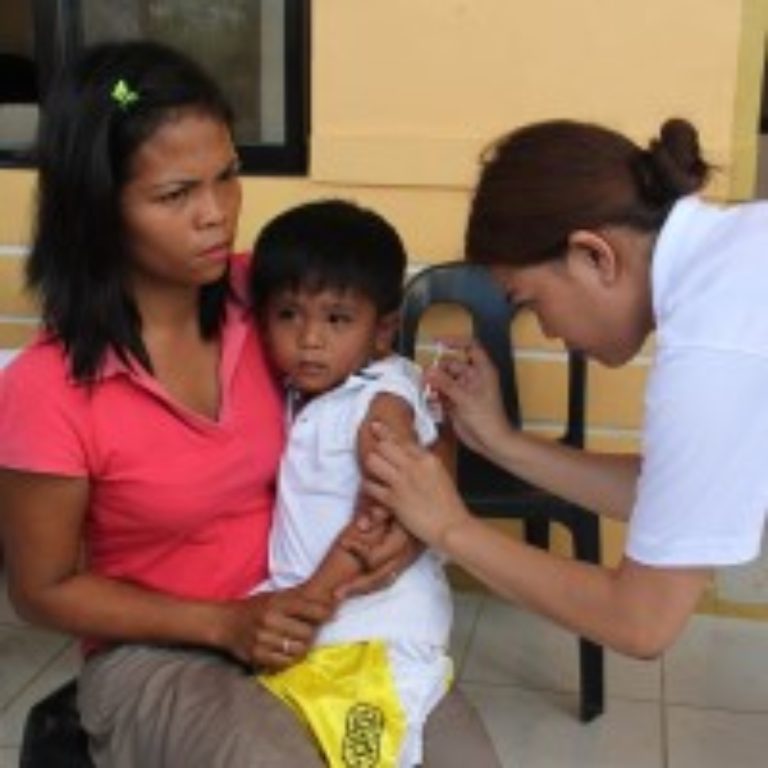
On Jan. 23, 2026, public-health authorities in Guinea-Bissau say that they have suspended a controversial US-funded hepatitis B…

On Jan. 23, 2026, chemists from the University of California, Los Angeles (UCLA) have found a big problem…
On Jan. 23, 2026, a case-control study published in The Journal of Pediatrics reports that routine childhood vaccinations,…

On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…