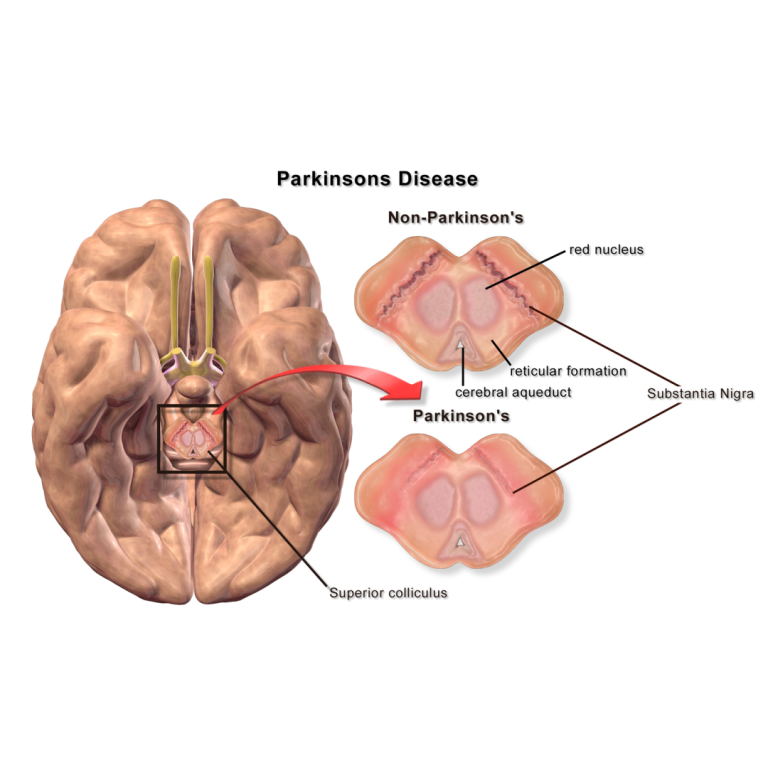
New stem cell treatment may offer hope for Parkinson’s disease
On Feb. 4, 2026, Keck Medicine of the University of Southern California (USC) announced it is conducting an early phase clinical…
On Feb. 4, 2026, Keck Medicine of the University of Southern California (USC) announced it is conducting an early phase clinical…
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…

On Jan. 28, 2026, U.S. drugmaker Eli Lilly announces signing an agreement worth up to $1.12 billion with…
On Jan. 28, 2026, the U.S. Environmental Protection Agency (EPA) launched the first step of its expedited review…

On Jan. 28, 2026, researchers at Google DeepMind have unveiled their latest artificial intelligence tool and claimed it…
On Jan. 27, 2026, a University of Cambridge study shows menopause is linked to reductions in grey matter…

On Jan. 23, 2026, chemists from the University of California, Los Angeles (UCLA) have found a big problem…
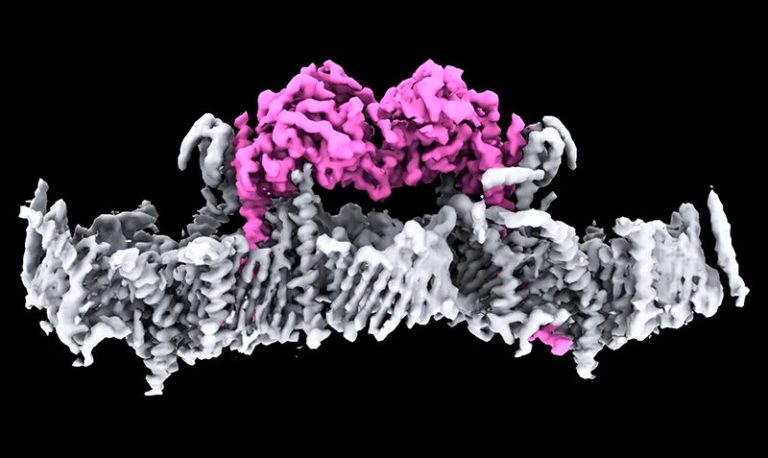
On Jan. 22, 2026, researchers backed by a Knight Initiative for Brain Resilience Catalyst Momentum Award have laid out…

On Jan. 21, 2026, a Johns Hopkins University-led team demonstrated robust connections between specific variations in a mother’s…
On Jan. 21, 2026, in a world first, a research team led by the University of Oxford’s Department…
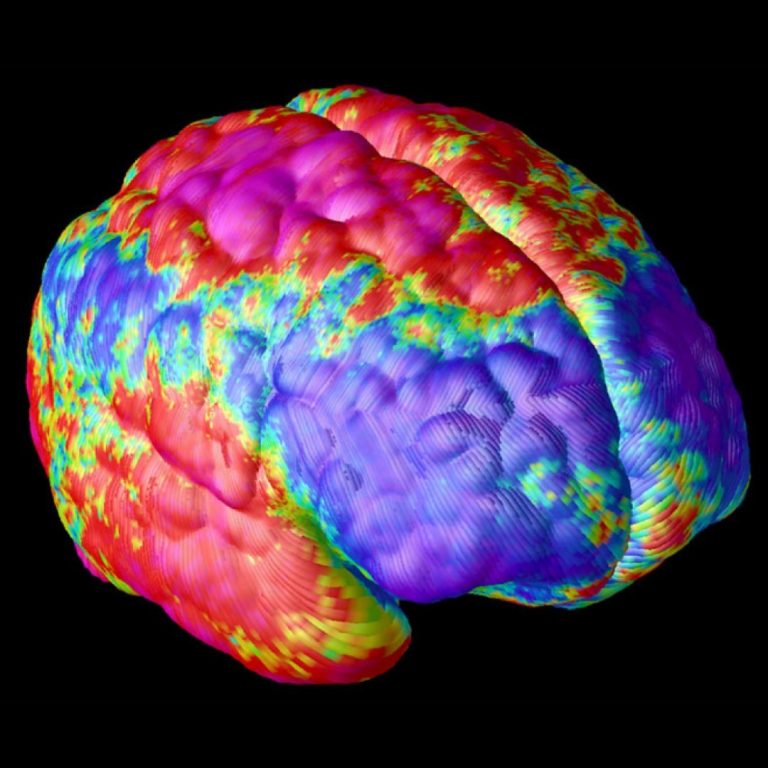
On Jan. 20, 2026, researchers announced they have identified two biomarkers — verbal learning and identifying other peoples’…
On Jan. 15, 2026, a team of Case Western Reserve University researchers announced they have discovered a particular…

On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…

On Jan. 13, 2026, collaborating with Google’s top artificial intelligence labs, a team of Yale researchers announced they…

On Jan. 13, 2026, The American Cancer Society (ACS) released Cancer Statistics, 2026, the organization’s annual report on cancer facts and trends. The findings show, for…

On Jan. 12, 2026, AbbVie announced a voluntary agreement with the Trump administration to further advance access and…

On Jan. 12, 2026, AbbVie and West Pharmaceutical Services announced a definitive agreement for AbbVie to acquire a…

On Jan. 9, 2026, Novartis announced plans to build its fourth US radioligand therapy (RLT) manufacturing facility in…
On Jan. 9, 2026, scientists at the Icahn School of Medicine at Mount Sinai, in partnership with the Multiple Myeloma Research…
On Jan. 8, 2026, for the first time, researchers at the University of British Columbia have demonstrated how…

On Jan. 8, 2026, Chinese drug makers’ licensing deals more than doubled in 2025 from a year earlier…

On Jan. 8, 2026, a major advance in cell biology has revealed how our cells safeguard their genetic…
On Jan. 8, 2026, researchers reported results from a study on the largest cohort of autobrewery syndrome (ABS)…

On Jan. 7, 2025, Eli Lilly and Ventyx Biosciences, a San Diego-based clinical-stage biopharmaceutical company focused on developing…

On Jan. 6, 2026, a systematic analysis for the Global Burden of Disease Global study shows that lower…

On Jan. 6, 2026, a study led by University of Washington School of Medicine researchers uncovered several new…

On Jan. 5, 2026, Novo Nordisk announced that the Wegovy® pill is now available, providing those seeking help…

On Dec. 22, 2025, Novo Nordisk announced announced that the US Food and Drug Administration (FDA) has approved…

On Dec. 21, 2025, Samsung Biologics announced that its wholly owned U.S. subsidiary, Samsung Biologics America, has entered…

On Dec. 19, 2025, Cytokinetics announced that the U.S. Food and Drug Administration (FDA) has approved MYQORZO™ (aficamten),…