Lilly selects Pennsylvania for new injectable medicine and device manufacturing facility
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…

On Jan. 28, 2026, U.S. drugmaker Eli Lilly announces signing an agreement worth up to $1.12 billion with…

On Dec. 11, 2025, the California Institute for Regenerative Medicine (CIRM) governing board approved funding of over $160…
On Nov. 17, 2025, in a major step forward for cancer care, researchers at ChristianaCare’s Gene Editing Institute…

On Oct. 24, 2025, Eli Lilly and Adverum Biotechnologies announced a definitive agreement for Lilly to acquire Adverum,…

On Sept. 24, 2025, a global clinical trial for a new Huntington’s disease treatment has posted positive results,…

On Sept. 14, 2025, scientists from the Gray Faculty of Medical & Health Sciences at Tel Aviv University…

On Jul. 18, 2025, the U.S. Food and Drug Administration (FDA) announced it has placed Sarepta Therapeutics investigational…
On May 29, 2025, The California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On May 15, 2025, a team at Children’s Hospital of Philadelphia (CHOP) and Penn Medicine announced that a…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…

On Sep. 10, 2024, researchers at the Karolinska Institute in Sweden announced a study that shows that gene…
On Aug. 2, 2024, the U.S. Food and Drug Administration approved Adaptimmune’s Tecelra (afamitresgene autoleucel), a gene therapy…

On Jul. 25, 2024, Pfizer announced that the European Commission (EC) has granted conditional marketing authorization for DURVEQTIX®…

On Jun. 28, 2024, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Jun. 20, 2024, the U.S. Food and Drug Administration expanded the approval of Sarepta Therapeutics’ Elevidys (delandistrogene…

On May 30, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had awarded $53 million to…

On Apr. 26, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BEQVEZ™ (fidanacogene…

On Mar. 18, 2024, the U.S. Food and Drug Administration (FDA) approved Orchard Therapeutics’ Lenmeldy (atidarsagene autotemcel), the…

On Jan. 16, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved CRISPR Therapeutics’ CASGEVY…

On Jan. 15, 2024, CSL announced that Swissmedic had authorised HEMGENIX® (etranacogene dezaparvovec), the first and currently only gene…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…
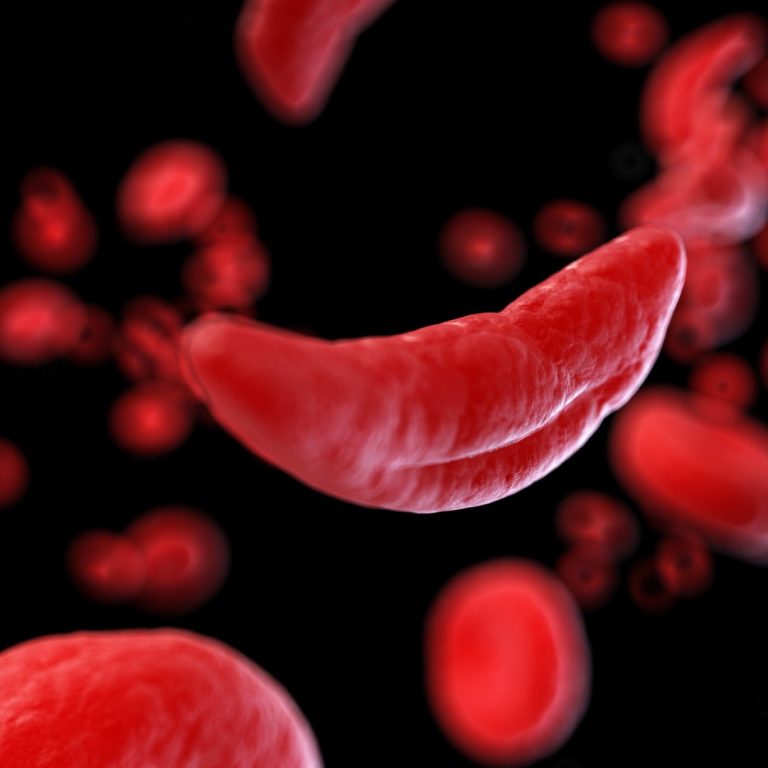
On Dec. 8, 2023, the U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia,…

On Nov. 16, 2023, Vertex and CRISPR Therapeutics announced that the United Kingdom (U.K.) Medicines and Healthcare products…

On Sept. 29, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $43.8 million to fund projects aimed…

On Aug. 24, 2023, researchers at City of Hope announced were awarded $32.3 million from the California Institute…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…

On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…
On Jun. 27, 2023, researchers from Tel Aviv University announced they have developed an innovative gene therapy that…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…