Lilly selects Pennsylvania for new injectable medicine and device manufacturing facility
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…
On Jan. 30, 2026, Eli Lilly announced plans to invest more than $3.5 billion in a new manufacturing…

On Jan. 5, 2026, Novo Nordisk announced that the Wegovy® pill is now available, providing those seeking help…

On Dec. 22, 2025, Novo Nordisk announced announced that the US Food and Drug Administration (FDA) has approved…

On Dec. 18, 2025, new national and state data was released by the Partnership to Fight Chronic Disease…
On Dec. 18, 2025, Novo Nordisk announced the submission of a New Drug Application (NDA) to the U.S….

On Dec. 1, 2025, the World Health Organization (WHO) has released its first guideline on the use of…

On Nov. 6, 2025, Eli Lilly announced an agreement with the U.S. government to expand access to its…

On Nov. 6, 2025, Novo Nordisk announced it has agreed with the U.S. Administration to lower drug prices…

On Oct. 5, 2025, Eli Lilly ill invest more than $1 billion in India in the coming years…

On Sept. 22, 2025, Pfizer and Metsera announced the companies have entered into a definitive agreement under which…
On Sept. 17, 2025, The New England Journal of Medicine published the results from the OASIS 4 phase…

On Aug. 28, 2025, Teva Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch…

On Aug. 25, 2025, Genentech, a member of the Roche Group broke ground on its newest U.S. manufacturing site…

On Aug. 18, 2025, Novo Nordisk announced the launch of a new offer that enables self-paying, eligible, type…

On Aug. 14, 2025, Eli Lilly has signed a deal worth $1.3 billion with privately held Superluminal Medicines…

On Jun. 22, 2025, The New England Journal of Medicine (NEJM) published results from Novo Nordisk’s phase 3…

On May 23, 2025 a Boston University School of Public Health (BUSPH) led study revealed that there were…

On May 19, 2025, researchers at the Broad Institute Ben Neale and Mark Daly are co-leading the study,…

On May 15, 2025, researchers at the Translational Genomics Research Institute (TGen), part of City of Hope, announced…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…
On Jan. 13, 2025, a study led by NYU Langone Health shows that the risk of developing dementia at…

On Dec. 20, 2024, the U.S. Food and Drug Administration (FDA) approved Eli Lilly’s Zepbound (tirzepatide) for the…

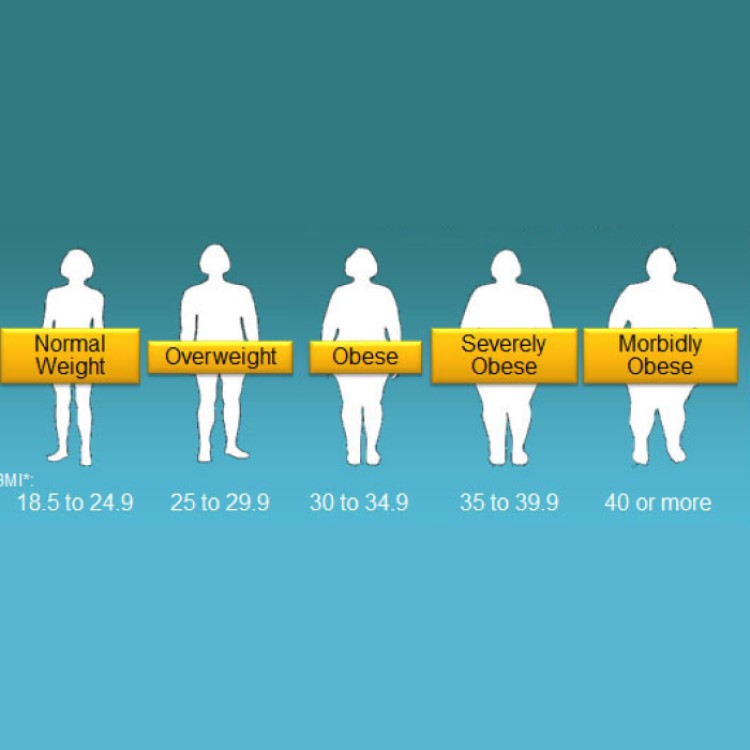
On Dec. 19, 2024, the U.S. Food and Drug Administration announced it had updated the definition of “healthy…

On Nov. 21, 2024, teams at the University of Texas Southwestern Medical Center report a new way that…
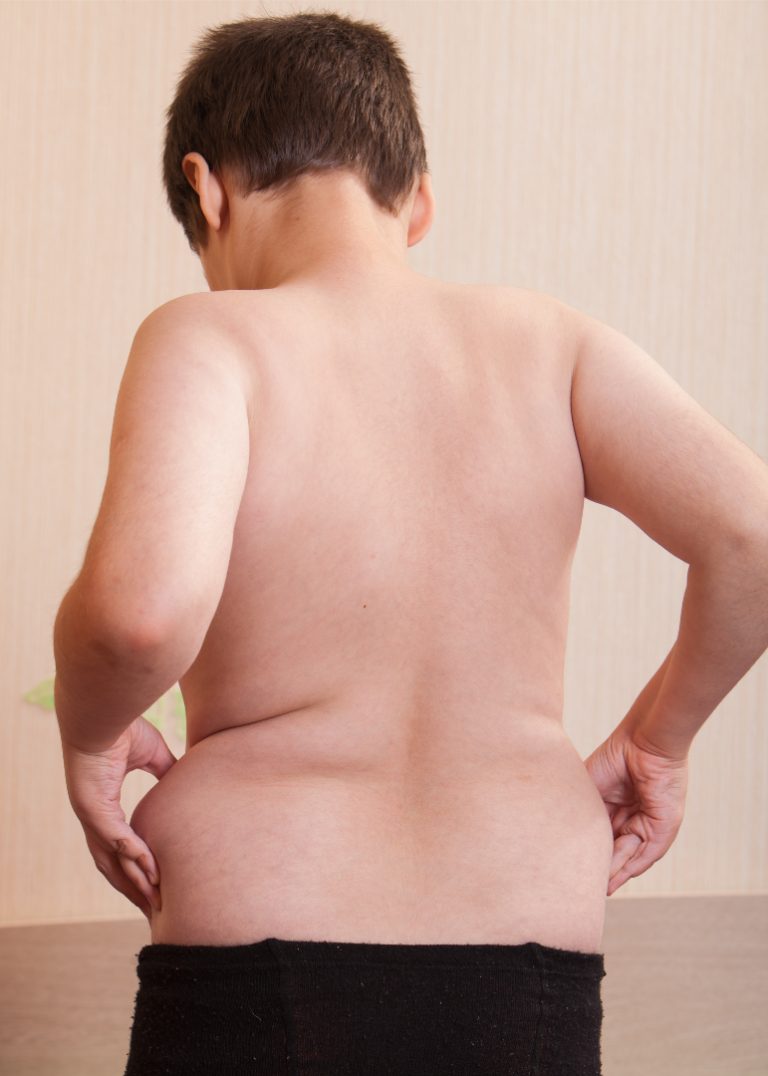
On Nov. 14, 2024, an analysis from the Global Burden of Disease Study Collaborator Network warned of an…

On Sep. 13, 2024, a Cleveland Clinic study was announced that identified key factors that can impact the…

On Aug. 13, 2024, Eli Lilly announced the opening of the Lilly Seaport Innovation Center (LSC), a research…

On Jun. 24, 2024, Novo Nordisk announced plans to invest 4.1 billion US dollars to build a second…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…

On Nov. 8, 2023, the U.S. Food and Drug Administration approved Eli Lilly’s Zepbound (tirzepatide) injection for chronic…