Immune Regulation partnered with DynPort Vaccine to advance drug product for COVID-19 into clinical trials
On Jul. 9, 2020, Altimmune announced that it had entered into a teaming agreement with DynPort Vaccine Company…

On Jul. 9, 2020, Altimmune announced that it had entered into a teaming agreement with DynPort Vaccine Company…

On Jul. 6, 2020, Emergent BioSolutions announced a five-year manufacturing services agreement with Janssen Pharmaceuticals, a Johnson &…
On Jul. 6, 2020, Anixa Biosciences announced that it and partner OntoChem had completed the initial in silico…

On Jul. 6, 2020, AIM ImmunoTech announced that it had signed a material transfer and research agreement with…

On Jul. 6, 2020, ADMA Biologics announced the commencement of operations and initiation of collections at its newest…
On Jul. 6, 2020, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) had granted Emergency…

On Jul. 6, 2020, Regeneron announced initiation of late-stage clinical trials evaluating REGN-COV2, Regeneron’s investigational double antibody cocktail…
On Jul. 6, 2020, Meridian HSN announced it had partnered with Moto-Para and Todos Medical to provide a…

On Jul. 6, 2020, Mylan announced that the Drug Controller General of India (DCGI) has approved its remdesivir…
On Jul. 4, 2020, the World Health Organization (WHO) accepted the recommendation from the Solidarity Trial’s International Steering…

On Jul. 3, 2020, Gilead Sciences announced the European Commission had granted conditional marketing authorization for Vekluryᆴ (remdesivir)…

On Jul. 3, 2020, CytoDyn announced it had signed an exclusive Distribution and Supply Agreement with American Regent…

On Jul. 2, 2020, Abivax announced that the first patient has been treated in its Phase 2b/3 study…

On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
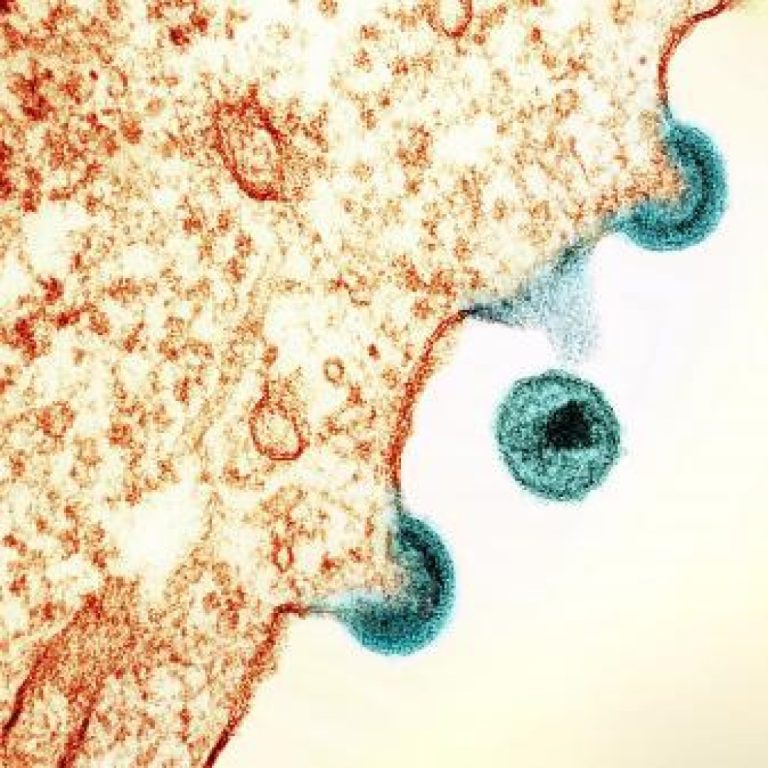
On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) approved Rukobia (fostemsavir), a new type of…
On Jul. 2, 2020, the U.S. Centers for Disease Control and Prevention (CDC) announced a publication in the…

On Jul. 2, 2020, Royal Philips announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Jul. 2, 2020, in response to the continuing need for more COVID-19 diagnostic tests, the Translational Genomics…

On Jul. 2, 2020, scientists from Duke University, Los Alamos National Laboratory and La Jolla Institute for Immunology…

On Jul. 2, 2020, UConn announced a licensing deal with Connecticut Biotech, a startup company headquartered in South…

On Jul. 2, 2020, Maxim Biomedical announced plans to produce and market as many as 3 million Maxim…

On Jul. 2, 2020, ViiV Healthcare, a GSK company, with Pfizer and Shionogi Limited, announced the FDA had…

On Jul. 2, 2020, Regeneron and Sanofi announced that the U.S. Phase 3 trial of Kevzara (sarilumab) 400…

On Jul. 2, 2020, Ceres Nanosciences and its collaborators at George Mason University announced they had have developed…
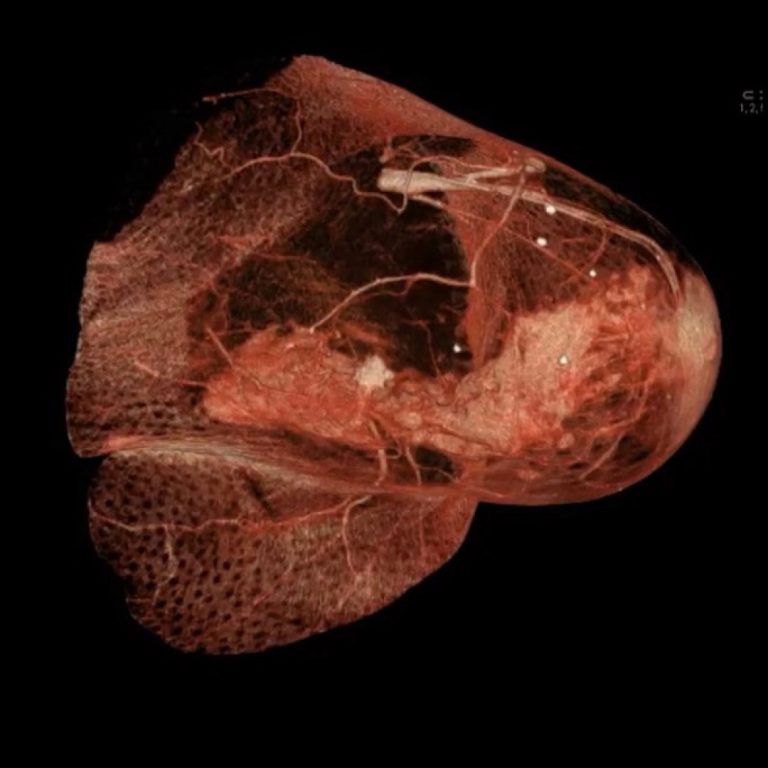
On Jul. 1, 2020, Intravacc announced the signing of a strategic partnership agreement with Dutch based CimCure, focusing…
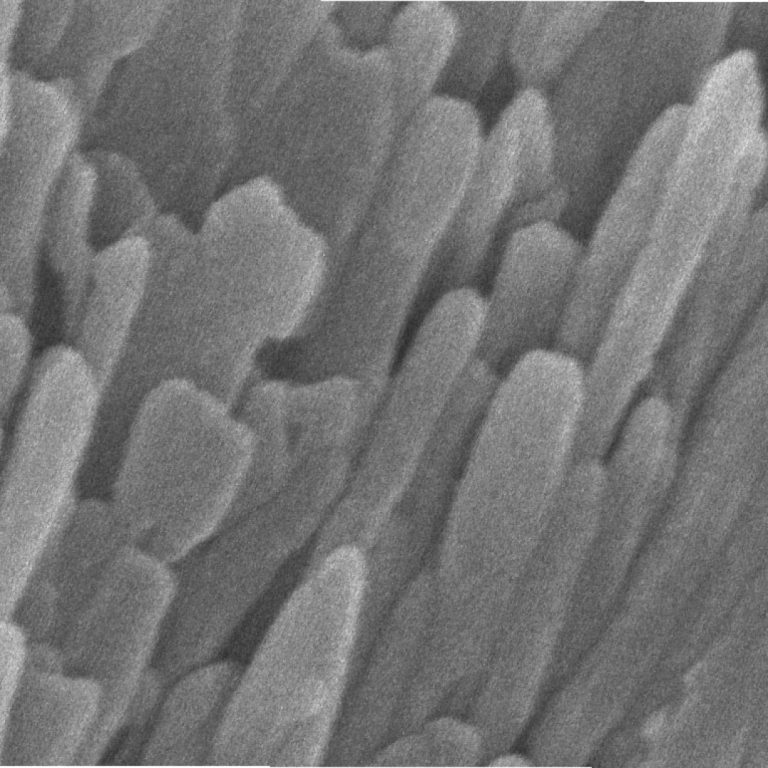
On Jul. 1, 2020, National Institutes of Health (NIH) funded scientists announced using a combination of advanced microscopy…

On Jul. 1, 2020, in a Perspective for the New England Journal of Medicine, members of the NIHメs…

On Jul. 1, 2020, FUJIFILM announced a partnership with Dr. Reddy’s Laboratories, a global pharmaceutical company headquartered in…

On Jul. 1, 2020, MediciNova announced that the Investigational New Drug Application (IND) for MN-166 (ibudilast) for prevention…

On Jul. 1, 2020, Merck and and and Ridgeback Biotherapeutics announced that the U.S. Federal Trade Commission (FTC)…