FDA approves updated label for Lilly’s Kisunla (donanemab-azbt) with new dosing in early symptomatic Alzheimer’s disease
On Jul. 9, 2025, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) has approved a…

On Jul. 9, 2025, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) has approved a…
On Jul. 9, 2025, pharmaceutical giant Merck is buying Verona Pharma, a company that focuses on respiratory diseases,…

On Jul. 8, 2025, the Chan Zuckerberg Initiative (CZI) and the Innovative Genomics Institute (IGI) announced the funding of the…

On Jul. 7, 2025, Australian scientists, including at the Charles Perkins Centre, University of Sydney, have successfully developed…

On Jul. 7, 2025, KalVista Pharmaceuticals announced that the U.S. Food and Drug Administration(FDA) has approved EKTERLY® (sebetralstat),…

On Jul. 4, 2025, the World Health Organization (WHO) released the Integrated Guidelines on the Clinical Management of…

On Jun. 30, 2025, AbbVie and Capstan Therapeutics announced a definitive agreement under which AbbVie will acquire Capstan,…

On Jun. 30, 2025, as in something out of science fiction, a team of researchers reached into the…

On Jun. 30, 2025, a University of Oxford led study reported that a drug used for Parkinson’s disease…
On Jun. 30, 2025, Fluoride was reintroduced into the drinking water in Alberta’s largest city nearly four years…

On Jun. 30, 2025, the Broad Institute announced that spatial mapping reveals genes contributing to fibrosis, or scarring,…

On Jun. 27, 2025, today is World Microbiome Day and the Microbiota Vault, together with all participating Local…

On Jun. 26, 2025, a team of UK-based scientists announced they are developing technology to create the first…

On Jun. 25, 2025, the National Institutes of Health (NIH) moved to reinstate about 900 grants that a…

On Jun. 22, 2025, The New England Journal of Medicine (NEJM) published results from Novo Nordisk’s phase 3…

On Jun. 20, 2025, the US Food and Drug Administration (FDA) announced it has approved Dupixent (dupilumab) for…

On Jun. 20, 2025, Vertex Pharmaceuticals announced publication of updated data from the Phase 1/2 portion of the…

On Jun. 18, 2025, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) has approved Yeztugo®…

On Jun. 18, 2025, SandboxAQ, an artificial intelligence startup spun out of Alphabet’s Google and backed by Nvidia…
On Jun. 18, 2025, federal health officials announced a listeria food poisoning outbreak that has killed three people…

On Jun. 17, 2025, Eli Lilly and Verve Therapeutics, a Boston-based clinical-stage company developing genetic medicines for cardiovascular…

On Jun. 16, 2025, Supernus Pharmaceuticals and Sage Therapeutics announced a definitive agreement for Supernus to acquire Sage…

On Jun. 16, 2025, the Allen Institute announced that for the first time ever, scientists have developed a…
On Jun. 16, 2025, BrainStorm Cell Therapeutics announced new survival data from 10 participants in its Expanded Access…
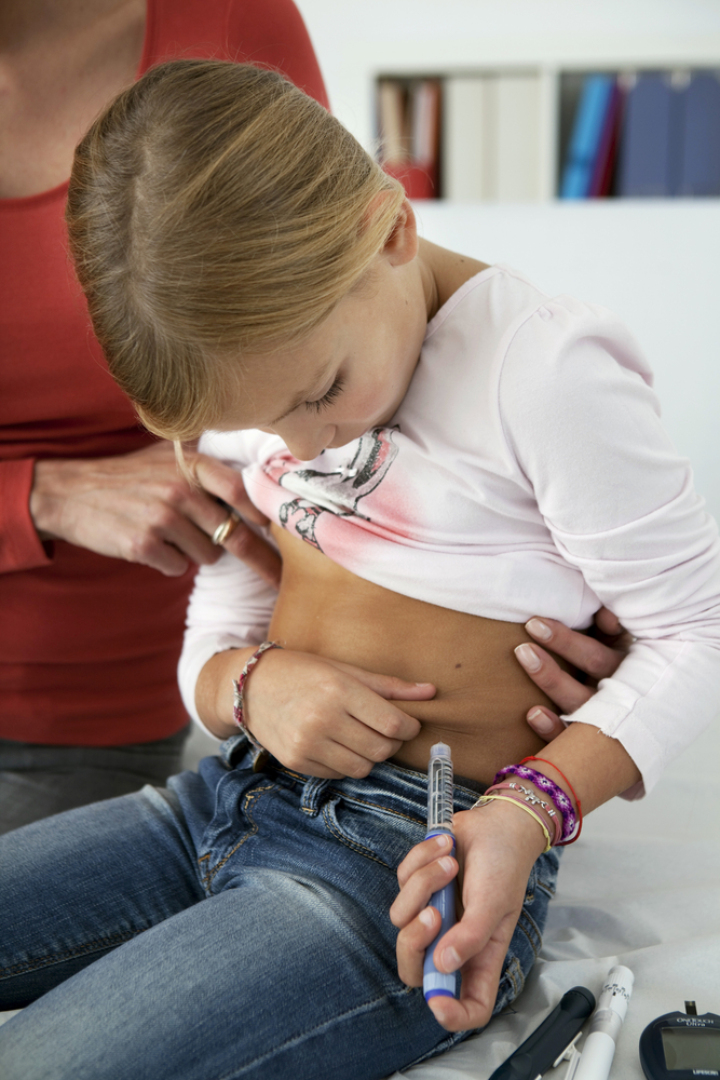
On Jun. 16, 2025, the National Institute for Health and Care Excellence (NICE) announced that the recommendation in…
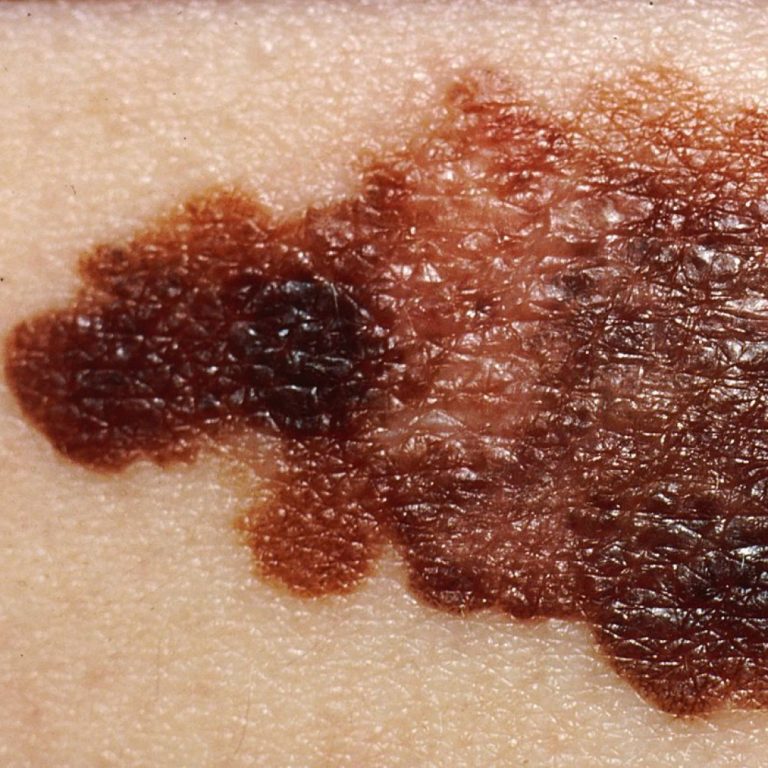
On Jun. 13, 2025, people in England will become the first in the world to receive belantamab mafodotin…

On Jun. 12, 2025, the Wellcome Sanger Institute, Parse Biosciences and the Computational Health Center at Helmholtz Munich…
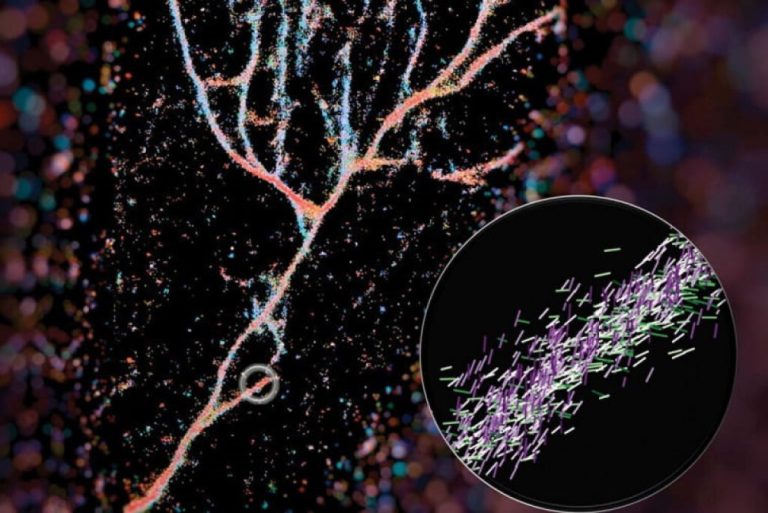
On Jun. 11, 2025, researchers from the Wellcome Sanger Institute, Centre of Genomic Regulation (CRG) and Institute for…

On Jun. 11, 2025, researchers at the University of California, Davis, announced they have developed an investigational brain-computer…
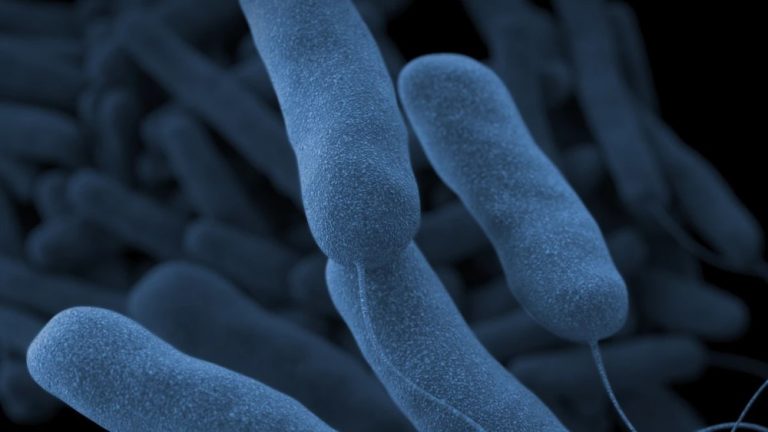
On Jun. 10, 2025, two patients treated within the past two months at the University of Washington Medical…