The Democratic Republic of the Congo declareed 14th Ebola outbreak over
On Jul. 4, 2022, the Democratic Republic of the Congo declared the end of the Ebola outbreak that…
On Jul. 4, 2022, the Democratic Republic of the Congo declared the end of the Ebola outbreak that…
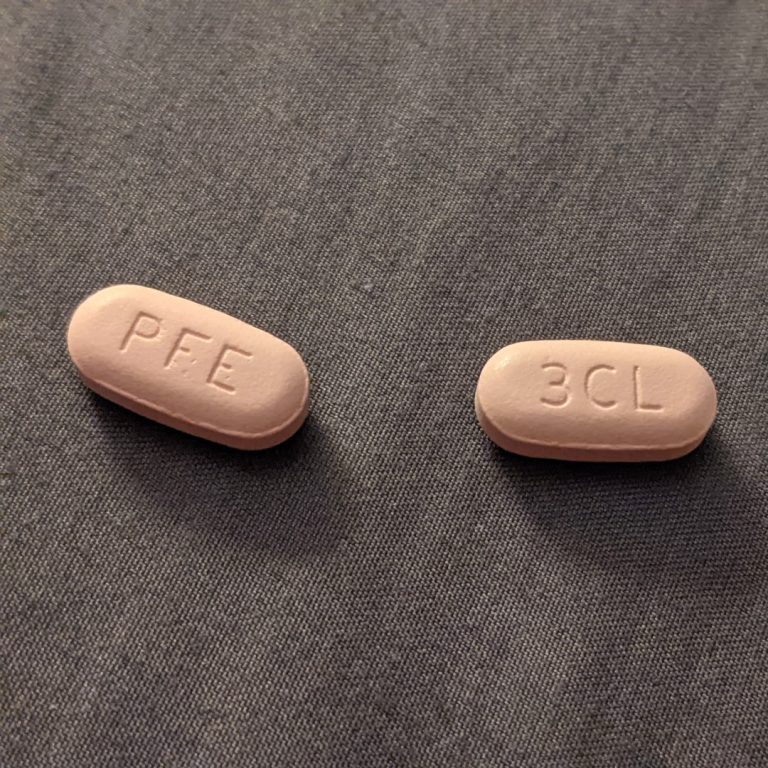
On Jun. 30 2022, Pfizer announced the submission of a New Drug Application (NDA) to the U.S. Food…

On Jun. 30, 2022, Pfizer and BioNTech announced a vaccine supply agreement with the U.S. government to support…

On Jun. 30, 2022, BD (Becton, Dickinson) announced that the BD MAX Respiratory Viral Panel (RVP), a new…
On Jun. 28, 2022, the National Institutes of Health (NIH) announced that a Phase 1 clinical trial of…
Play the COVID-19 Wheel of Fortune and see how lucky you are! You have two wheels to choose…

On Jun. 24 2022, Pfizer and BioNTech announced positive data evaluating the safety, tolerability, and immunogenicity of two…

On Jun. 24, 2022, Emergent BioSolutions announced that the U.S. Food and Drug Administration accepted for review the…

On Jun. 23 2022, Novavax announced the filing of a Supplement to a New Drug Submission with Health…
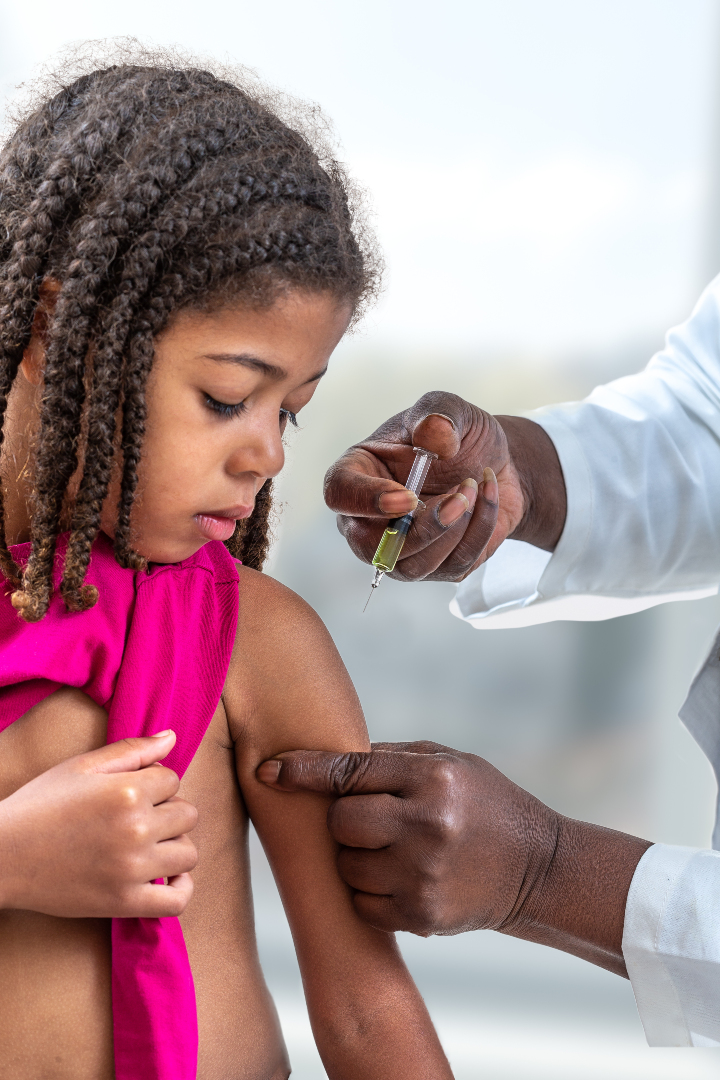
On Jun. 23 2022, Novavax announced that the Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional…

On Jun. 23 2022, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…

On Jun. 23, 2022, BioNTech announced it had reached a milestone in the establishment of scalable mRNA vaccine…
On Jun. 23, 2022, Innovation Pharmaceuticals reported that Brilacidin, the Company’s defensin-mimetic drug candidate exhibiting broad-spectrum antiviral activity,…

On Jun. 22, 2022, Merck announced that that the U.S. Food and Drug Administration (FDA) had approved an…

On Jun. 21, 2022, Anixa Biosciences announced the publication of a peer-reviewed journal article in Clinical and Experimental…

On Jun. 17, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Jun. 17, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration had granted emergency use…

On Jun. 17, 2022, Benaroya Research Institute at Virginia Mason (BRI) announced a 5-year, $11.4 million grant from…
On Jun, 15, 2022, a multidisciplinary team of scientists, announced they had obtained and studied ancient Y. pestis…

On Jun. 15, 2022, Pfizer and BioNTech announced the European Medicines Agency (EMA) had initiated a rolling review…

On Jun. 15, 2022, Roche announced that the US Food and Drug Administration had issued Emergency Use Authorization…
On Jun. 14 2022, Oragenics announced the publication of an article co-authored by Oragenics and collaborators at Inspirevax…

On Jun. 14 2022, Pfizer reported data from the Phase 2/3 EPIC-SR (Evaluation of Protease Inhibition for COVID-19…

On Jun. 13 2022, Novavax announced that the Australian Therapeutic Goods Administration (TGA) had granted provisional registration of…

On Jun. 10, 2022, the U.S. Department of Agriculture’s (USDA) National Veterinary Services Laboratory (NVSL) confirmed the presence…

On Jun. 10, 2022, researchers from Case Western Reserve School of Medicine, Case Comprehensive Cancer Center, and The…

On Jun. 10, 2022, CDC and public health officials in several states announced they were investigating multistate outbreaks…

On Jun. 8, 2022, Moderna announced new clinical data on its Omicron-containing bivalent COVID booster candidate, mRNA-1273.214, containing…

On Jun. 7, 2022, Zoetis announced an agreement to acquire Basepaws, a privately held petcare genetics company, which…

On Jun. 7, 2022, Merck and Ridgeback Biotherapeutics announced the Annals of Internal Medicine had published additional data…