Gates Foundation Announces Catalytic Funding to Spark New Era of Women-Centered Research and Innovation
On Aug. 4, 2025, The Gates Foundation announced a $2.5 billion commitment through 2030 to accelerate research and…

On Aug. 4, 2025, The Gates Foundation announced a $2.5 billion commitment through 2030 to accelerate research and…

On Aug. 4, 2025, The Florida Department of Health said on Monday that there have been 21 cases…

Aug. 1, 2025, Mallinckrodt and Endo announced that they have completed their merger to create a global, scaled,…
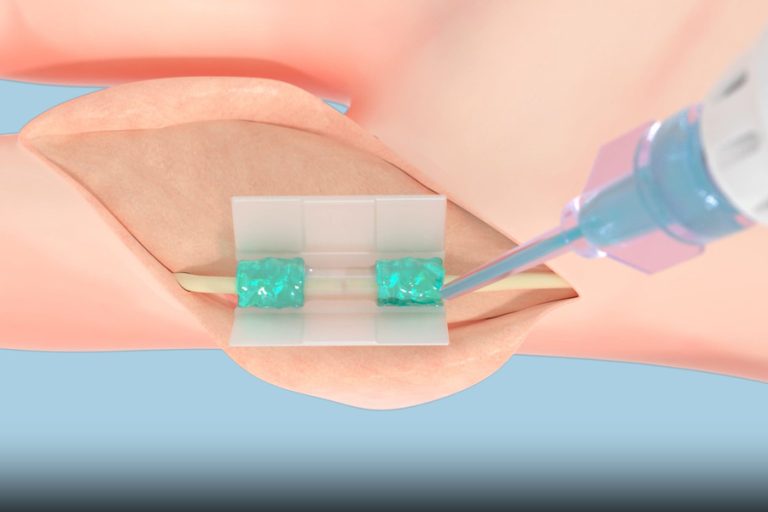
On Aug. 1, 2025, Tissium announced it has begun offering surgeons a new solution based on a biopolymer…
On Jul. 31, 2025, Novo Nordisk announced that the US Food and Drug Administration (FDA) approved Alhemo® (concizumab-mtci)…

On Jul. 31, 2025, Susan Monarez, Ph.D., was sworn in as Director of the U.S. Centers for Disease…

On Jul. 31, 2025, Biogen announced it intends to invest an additional $2 billion in its existing manufacturing footprint…
On Jul. 30, 2025, a study led by Northwestern Medicine shows that most U.S. adults have a “heart…

On Jul. 29, 2025, Stanford Medicine researchers announced the creation a team of virtual scientists backed by artificial…

On Jul. 29, 2025, Texas A&M study finds immersive VR nature experiences improve mood and quality of life….

On Jul. 29, 2025, two studies showed that influenza vaccine effectiveness (VE) in the United States was similar…

On Jul. 28, 2025, a study published in Nature Neuroscience shows that potential contact with approaching infectious avatars,…
On Jul. 28, 2025, as we mark World Hepatitis Day, the World Health Organization (WHO) calls on governments…

On Jul. 28, 2025, Kaiser Permanente Washington Health Research Institute (KPWHRI) and Kaiser Permanente Center for Health Research…

On Jul. 25, 2025, Pfizer and BioNTech announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products…

On Jul. 24, 2025, a University of Minnesota study shows that widespread immunity through vaccination or natural infection…
On Jul. 24, 2025, a team of researchers from TGen, part of City of Hope, and Baylor Scott…

On Jul. 24, 2025, The China Times reported that China is making inroads in the race for innovative…

On Jul. 24, 2025, an analysis of studies incorporating data from almost 30 million people has highlighted the…
On Jul. 24, 2025, researchers at the Broad Institute and the National Emerging Infectious Diseases Laboratories (NEIDL) at…
On Jul. 24, 2025, a world-first study led by Museums Victoria Research Institute has revealed that beneath the…

On Jul. 24, 2025, the U.S. Centers for Disease Control and Prevention (CDC) reported that during October 2023–March…

On Jul. 23, 2025, results from a newly published study highlight the growing spread of drug resistance across…
On Jul. 23, 2025, research at Tufts and elsewhere has revealed the eight essential nutrients that make up…

On Jul. 22, 2025, AstraZeneca announces $50 billion of investment in the United States by 2030, building on…

On Jul. 22, 2025, The World Health Organization (WHO) issued an urgent call for action on Tuesday to…

On Jul. 22, 2025, Sanofi announces it has entered into an agreement to acquire Vicebio, a privately held…

On Jul. 22, 2025, Health officials have confirmed a case of brain-eating amoeba in South Carolina. The state’s…

On Jul. 21, 2025, new research led by the Universities of Copenhagen and Bristol shows analysing genes at…

On Jul. 20, 2025, new detections of New World screwworm (NWS) in Mexico led U.S. Department of Agriculture (USDA)…