CytoDyn announced exclusive agreement for distribution and supply of leronlimab for treatment of COVID-19 in U.S.
On Jul. 3, 2020, CytoDyn announced it had signed an exclusive Distribution and Supply Agreement with American Regent…

On Jul. 3, 2020, CytoDyn announced it had signed an exclusive Distribution and Supply Agreement with American Regent…

On Jul. 2, 2020, Abivax announced that the first patient has been treated in its Phase 2b/3 study…

On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
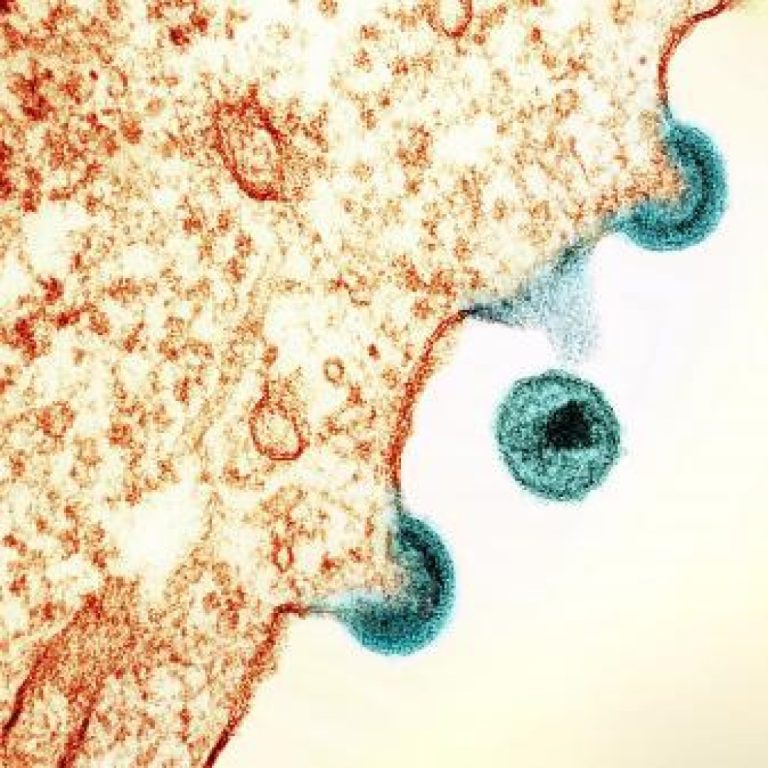
On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) approved Rukobia (fostemsavir), a new type of…
On Jul. 2, 2020, the U.S. Centers for Disease Control and Prevention (CDC) announced a publication in the…

On Jul. 2, 2020, Royal Philips announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Jul. 2, 2020, in response to the continuing need for more COVID-19 diagnostic tests, the Translational Genomics…

On Jul. 2, 2020, scientists from Duke University, Los Alamos National Laboratory and La Jolla Institute for Immunology…

On Jul. 2, 2020, UConn announced a licensing deal with Connecticut Biotech, a startup company headquartered in South…

On Jul. 2, 2020, Maxim Biomedical announced plans to produce and market as many as 3 million Maxim…

On Jul. 2, 2020, ViiV Healthcare, a GSK company, with Pfizer and Shionogi Limited, announced the FDA had…

On Jul. 2, 2020, Regeneron and Sanofi announced that the U.S. Phase 3 trial of Kevzara (sarilumab) 400…

On Jul. 2, 2020, Ceres Nanosciences and its collaborators at George Mason University announced they had have developed…
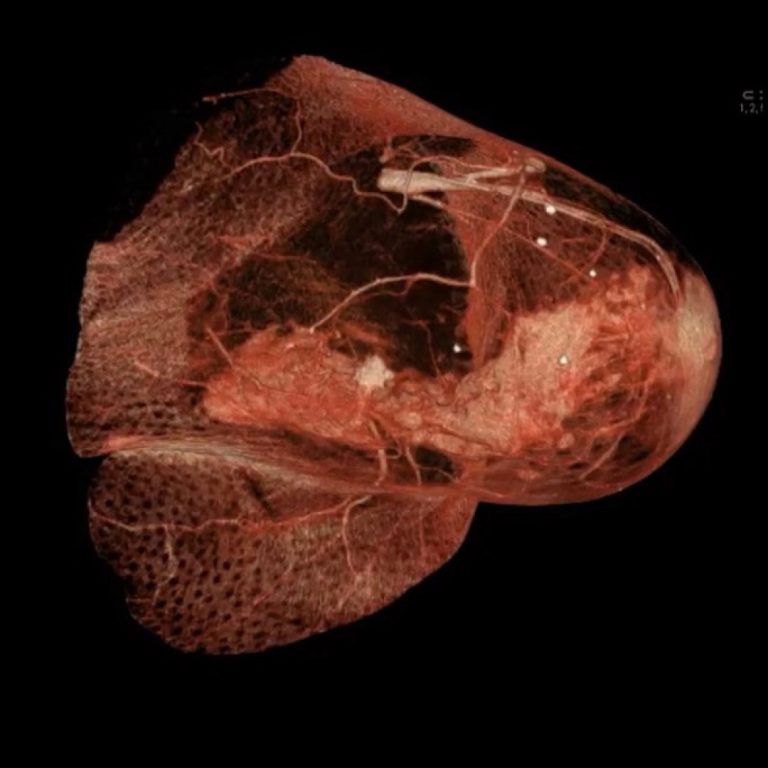
On Jul. 1, 2020, Intravacc announced the signing of a strategic partnership agreement with Dutch based CimCure, focusing…
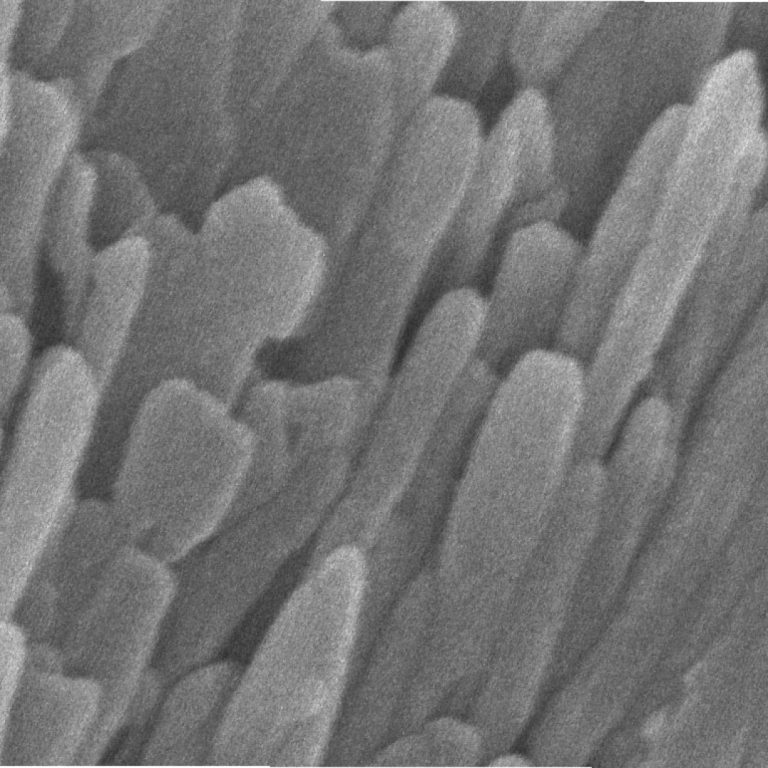
On Jul. 1, 2020, National Institutes of Health (NIH) funded scientists announced using a combination of advanced microscopy…

On Jul. 1, 2020, in a Perspective for the New England Journal of Medicine, members of the NIHメs…

On Jul. 1, 2020, FUJIFILM announced a partnership with Dr. Reddy’s Laboratories, a global pharmaceutical company headquartered in…

On Jul. 1, 2020, MediciNova announced that the Investigational New Drug Application (IND) for MN-166 (ibudilast) for prevention…

On Jul. 1, 2020, Merck and and and Ridgeback Biotherapeutics announced that the U.S. Federal Trade Commission (FTC)…

On Jul. 1, 2020, T2 Biosystems announced completion of validation of its COVID-19 molecular diagnostic test, the T2SARS-CoV-2…

On Jul. 1, 2020, OPKO Health announced the results of COVID-19 molecular PCR and antibody tests for nursing…

On Jul. 1, 2020, Pfizer and BioNTech announced preliminary data from the most advanced of four investigational vaccine…

On Jul. 1, 2020, InBios announced that it had received Emergency Use Authorization (EUA) from the FDA for…

On Jun. 30, 2020, scientists from the Innovative Genomics Institute (IGI) at the University of California Berkeley announced…

On Jun. 30, 2020, AquaBounty Technologies announced it had successfully commenced the commercial-scale harvest of conventional Atlantic salmon…

On Jun. 30, 2020, Altimmune announced dosing of the first patient in the Company’s Phase 1b clinical trial…

On Jun. 29, 2020, Gilead Sciences announced that the Covid-19 drug Remdesivir will cost $3,120 for typical patient…
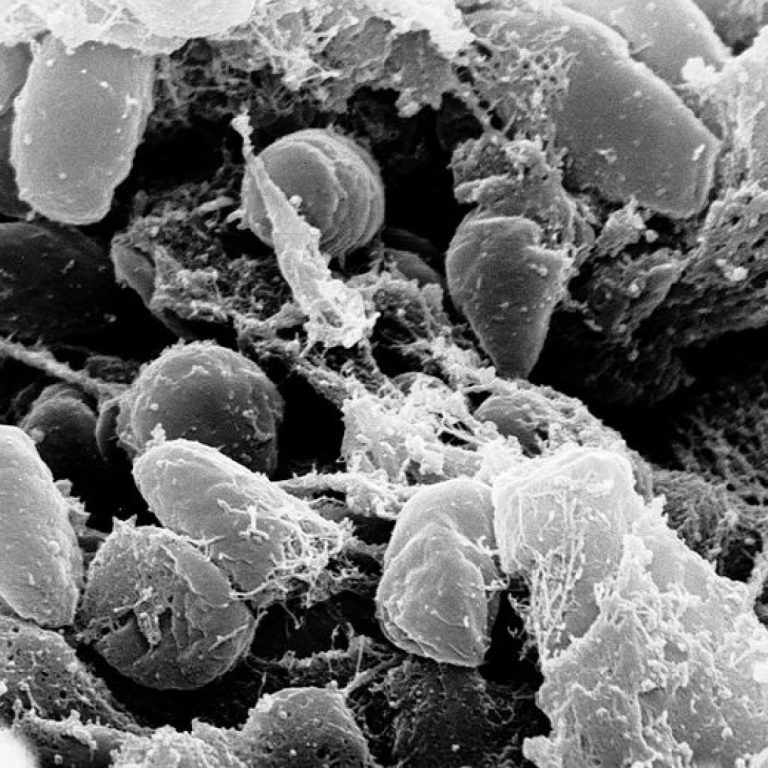
On Jun. 29, 2020, National Institutes of Health (NIH) researchers announced they had discovered that Mediterranean populations may…

On Jun. 29, 2020, the U.S. Dept. of Veterans Affairs (VA) announced it had launched a digital COVID-19…

On Jun. 29, 2020, the University of Oxford reported that potential COVID-19 hotspots can be identified using a…