Will China’s new chromosome editing tool unlock new wave of genetic advances?
On Aug. 10, 2025, a group of Chinese scientists has overcome a challenge that stumped biologists for decades…
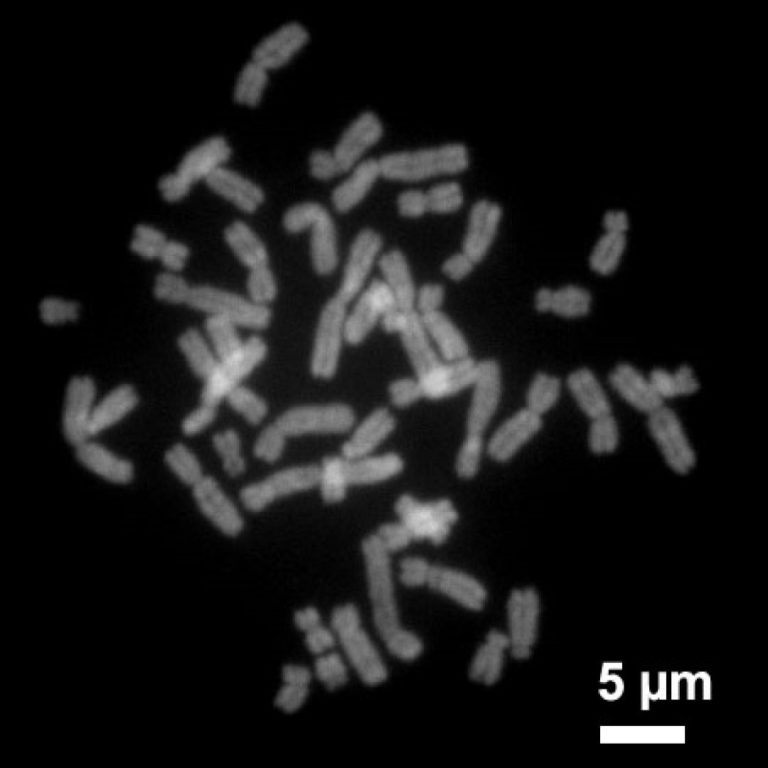
On Aug. 10, 2025, a group of Chinese scientists has overcome a challenge that stumped biologists for decades…
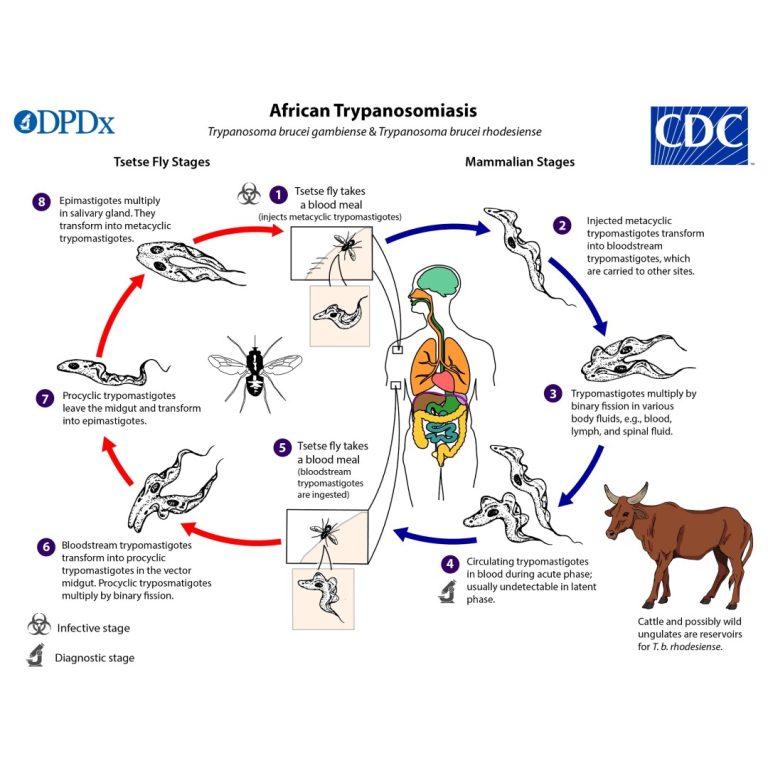
On Aug. 8, 2025, the World Health Organization (WHO) has validated Kenya as having eliminated human African trypanosomiasis…
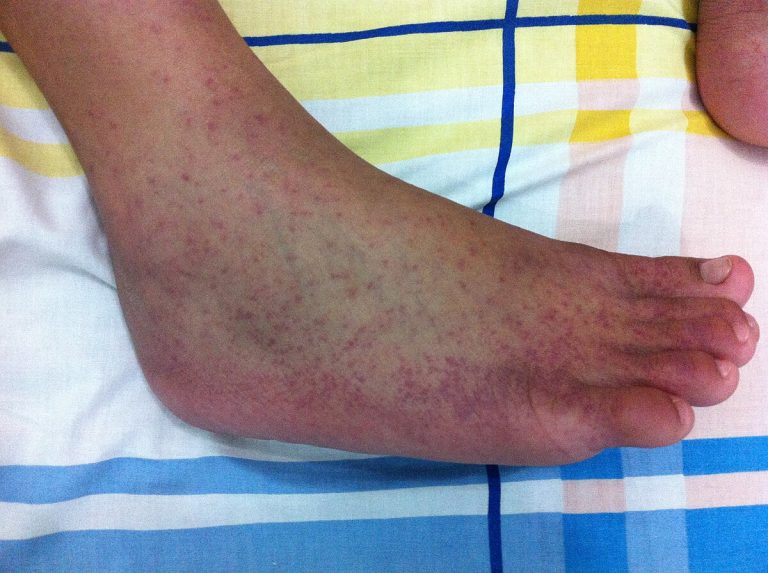
On Aug. 8, 2025, experts in mosquito-borne infectious diseases are warning against excessive insect eradication campaigns as cities…
On Aug. 7, 2025, The National Institute for Health and Care Excellence (NICE) has today announced the approval…

On Aug. 7, 2025, The first paper from a multi-year clinical research study has been published in The…
On Aug. 7, 2025, research scientists at Scripps Research announced a new platform that enables fast, scalable protein…

On Aug. 6, 2025, CareDx announced the publication of the Kidney Allograft Outcomes AlloSure Registry (KOAR) study in…

On Aug. 6, Mars has licensed Pairwise’s Fulcrum® gene editing tools for cacao research and development. This licensing…
On Aug. 6, 2025, Jazz Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Modeyso™ (dordaviprone)…
On Aug. 6, 2025, the Tacoma-Pierce County Health Department (TPCHD) reported an East Pierce County, Washington woman who…

On Aug. 5, 2025, a team of Chinese researchers led by Prof. GAO Caixia from the Institute of…

On Aug. 5, 2025, U.S. health officials are warning travelers headed to southern China of an outbreak of…

On Aug. 4, 2025, The Gates Foundation announced a $2.5 billion commitment through 2030 to accelerate research and…

On Aug. 4, 2025, The Florida Department of Health said on Monday that there have been 21 cases…
On Aug. 4, 2025, the New York City Health Department provided an update on the investigation into a…

Aug. 1, 2025, Mallinckrodt and Endo announced that they have completed their merger to create a global, scaled,…
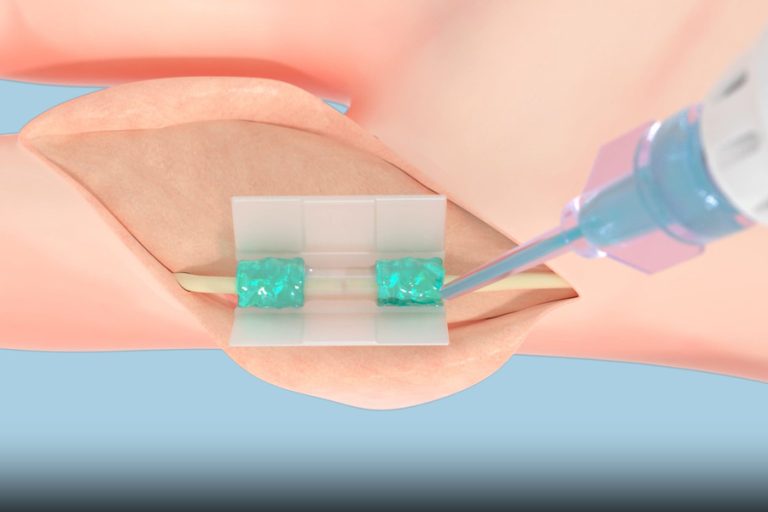
On Aug. 1, 2025, Tissium announced it has begun offering surgeons a new solution based on a biopolymer…
On Jul. 31, 2025, Novo Nordisk announced that the US Food and Drug Administration (FDA) approved Alhemo® (concizumab-mtci)…

On Jul. 31, 2025, Susan Monarez, Ph.D., was sworn in as Director of the U.S. Centers for Disease…

On Jul. 31, 2025, Biogen announced it intends to invest an additional $2 billion in its existing manufacturing footprint…
On Jul. 30, 2025, a study led by Northwestern Medicine shows that most U.S. adults have a “heart…

On Jul. 29, 2025, Stanford Medicine researchers announced the creation a team of virtual scientists backed by artificial…

On Jul. 29, 2025, Texas A&M study finds immersive VR nature experiences improve mood and quality of life….

On Jul. 29, 2025, two studies showed that influenza vaccine effectiveness (VE) in the United States was similar…

On Jul. 28, 2025, a study published in Nature Neuroscience shows that potential contact with approaching infectious avatars,…
On Jul. 28, 2025, as we mark World Hepatitis Day, the World Health Organization (WHO) calls on governments…

On Jul. 28, 2025, Kaiser Permanente Washington Health Research Institute (KPWHRI) and Kaiser Permanente Center for Health Research…

On Jul. 25, 2025, Pfizer and BioNTech announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products…

On Jul. 25, 2025, the New York City Health Department (Health Department) announced an investigation into a community…

On Jul. 24, 2025, a University of Minnesota study shows that widespread immunity through vaccination or natural infection…