Geneticists linked DNA of famed sled dog Balto to modern breeds
On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…

On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…

On Apr. 26, 2023, Vertex announced the Food and Drug Administration (FDA) had approved the expanded use of…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Apr. 24, 2023, Boehringer Ingelheim inaugurated its state-of-the-art Biologicals Development Center in Biberach an der Ri�, Germany….
On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 20, 2023, Massachusetts Institute of Technology (MIT) researchers announced that have developed machine-learning algorithms that can…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Apr. 17, 2023, a new initiative led by Jennifer Doudna and Jill Banfield at the Innovative Genomics…

On Apr. 17, 2023, the U.S. FDA approved Gamida Cell’s Omisirge (omidubicel-onlv), a substantially modified allogeneic (donor) cord…

On Apr. 15, 2023, the World Health Organization (WHO) announced that six additional laboratory-confirmed cases of Marburg virus…

On Apr. 14, 2023, Revance Therapeutics announced that the U.S. Food and Drug Administration (FDA) had approved the…

On Apr. 14, 2023, the National Health Commission of the People’s Republic of China reported a confirmed case…

On Apr. 14, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for the…

On Apr. 13, 2023, in an enormous leap forward in the understanding of Parkinson’s disease (PD), researchers announced…
On Apr. 11, 2023, a National Institutes of Health (NIH) clinical trial was stopped early because a daily…
On Apr. 10, 2023, the U.S. Department of Health and Human Services (HHS) announced it was spending over…
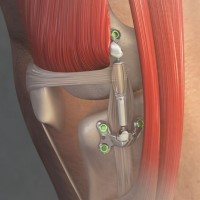
On Apr. 10, 2023, the U.S. Food and Drug Administration authorized for marketing Moximed’s MISHA Knee System, an…

On Apr. 7, 2023, the U.S. National Park Service reported Highly Pathogenic Avian Influenza (HPAI) had been confirmed…

On Apr. 5, 2023, researchers at Oregon Health & Science University’s OHSU Casey Eye Institute announced that gene…

On Apr. 5, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 5, 2023, the Food and Agriculture Organization (FAO), in collaboration with the World Health Organization (WHO),…

On Apr. 5, 2023, Aberdeen Proving Ground South, formerly known as Edgewood, celebrated the opening of the Department…
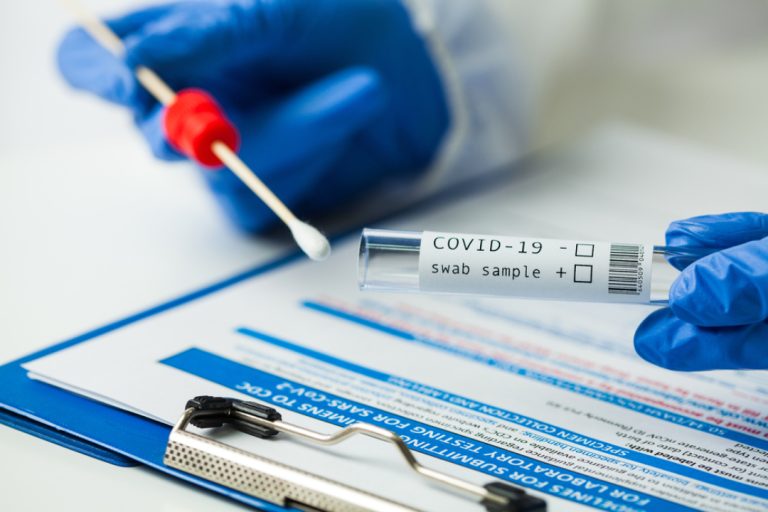
On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…

On Apr. 4, 2023, NanoViricides announced that it had executed a license agreement with Karveer Meditech, Kolhapur, India,…

On Apr. 3, 2023, a study from the National Eye Institute (NEI) identified rare genetic variants that could…

On Apr. 3, 2023, the U.S. Department of Health and Human Services (HHS) released a National Cancer Plan,…

On Mar. 31, 2023, the Oregon Department of Agriculture and the United States Department of Agriculture’s Animal and…
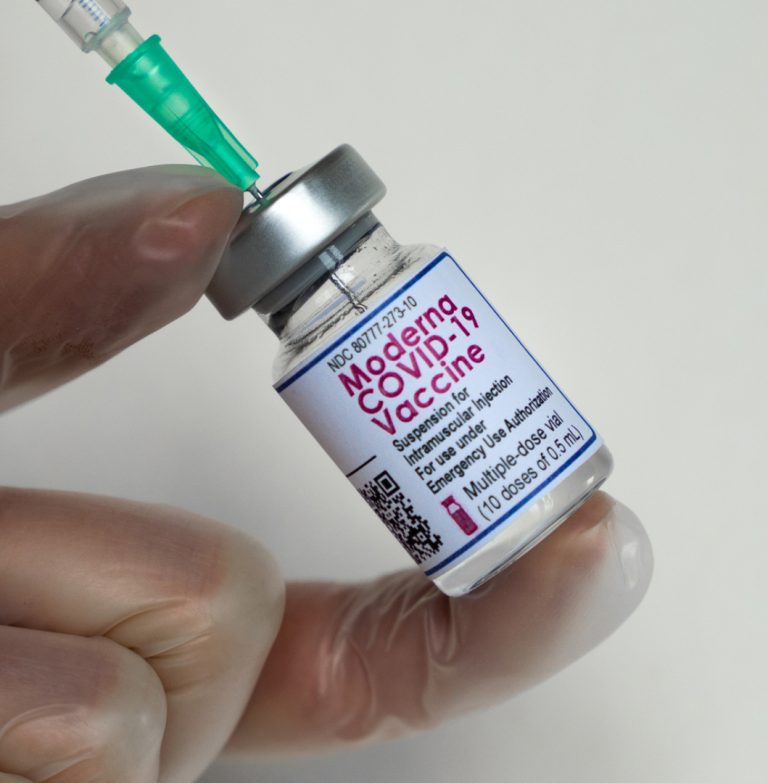
On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…

On Mar. 29, 2023, the mortality gap between the United States and other high-income nations substantially expanded during…