Scientists pinpointed mechanisms associated with severe COVID-19 blood clotting
On Feb. 17, 2022, after studying blood samples from 244 patients hospitalized for COVID-19, a group of researchers,…

On Feb. 17, 2022, after studying blood samples from 244 patients hospitalized for COVID-19, a group of researchers,…

On Feb. 17, 2022, Resverlogix announced that the first Brazilian site had been initiated for its Phase 2b…
On Feb. 16, 2022, Moderna announced that the Therapeutic Goods Administration in Australia had granted provisional registration for…

On Feb. 16, 2022, BioNTech announced it had introduced its approach to establishing scalable vaccine production by developing…

On Feb. 16, 2022, the McKelvey School of Engineering at Washington University in St. Louis, announced that it…

On Feb. 15, 2022, Pfizer announced that the European Medicines Agency had approved the companyメs 20-valent pneumococcal conjugate…
On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…
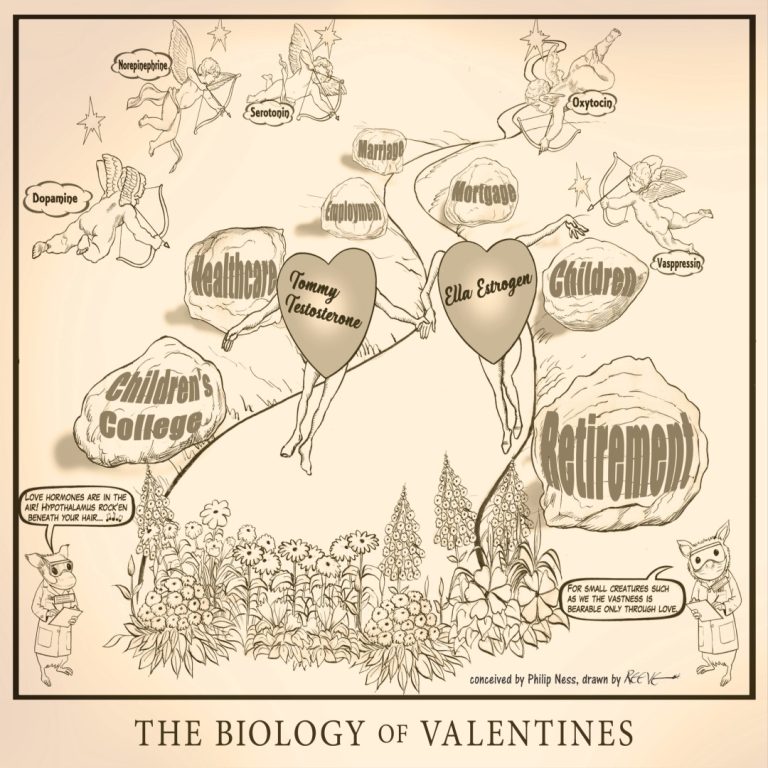
The Biology of Valentines illustrates the biological components of love combined with realities of life. Published Feb. 14,…

On Feb. 14, 2022, Novavax announced that the Singapore Health Sciences Authority (HSA) had issued interim authorization for…

On Feb. 14, 2022, Novavax announced its submission to Swissmedic, the Swiss Agency for Therapeutic Products, for conditional…

On Feb. 14, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 14, 2022, the U.S. Centers for Disease Control and Prevention (CDC) reported that the current risk…
On Feb. 12, 2022, China’s medical products regulator announced that it had given conditional approval for Pfizer’s COVID-19…

On Feb. 11, 2022, Pfizer and BioNTech announced plans to extend their rolling submission to the U.S. Food…

On Feb. 11, 2022, Roche announced that Actemraᆴ/RoActemraᆴ (tocilizumab) intravenous (IV) had been granted World Health Organization (WHO)…

On Feb. 11, 2022, the WHO announced the addition of tocilizumab, a monoclonal antibody, to its list of…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 10, 2022, Vir Biotech announced that preclinical data suggested that sotrovimab, an investigational monoclonal antibody authorized…

On Feb. 10, 2022, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, achieved its primary effectiveness…
On Feb. 10, 2022, Scripps Research announced that the COVID-causing virus SARS-CoV-2 harbors a vulnerable site at the…

On Feb. 9, 2022, the Indiana Department of Health confirmed that a case of H5N1 highly pathogenic avian…

On Feb. 8, 2022, Howard Hughes Medical Institute (HHMI) announced The program, named Codetta, can read the genome…
On Feb. 8, 2022, Moderna announced a new supply agreement with the government of Colombia for 10.8 million…

On Feb. 8, 2022, Merck and Ridgeback Biotherapeutics announced that a total of 3.1 million courses of molnupiravir,…

On Feb. 8, 2022, a research team led at Boston University School of Public Health reported on links…

On Feb. 8, 2022, researchers at Oxford University announced they had implanted a novel closed-loop research platform for…

On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…
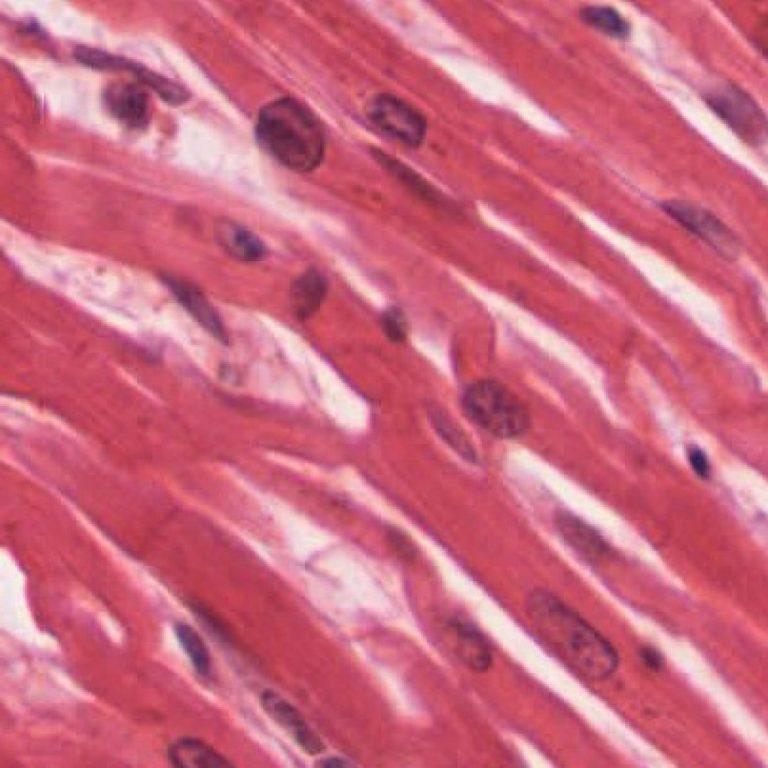
On Feb. 7, 2022, researchers at Washington University School of Medicine in St. Louis announced an analysis of…

On Feb. 7, 2022, scientists working with the Queen Mary University of London and the London Genome Centre…