European Commission approved Aimmune’s PALFORZIA as first-ever treatment for peanut allergy in the EU
On Dec. 21, 2020, Aimmune Therapeutics announced that the European Commission (EC) had approved PALFORZIA [defatted powder of…

On Dec. 21, 2020, Aimmune Therapeutics announced that the European Commission (EC) had approved PALFORZIA [defatted powder of…

On Dec. 21, 2020, Quidel announced that it had received Emergency Use Authorization from the U.S. Food and…

On Dec. 19, 2020, after a transparent, evidence-based review of available data, the Advisory Committee on Immunization Practices…

On Dec. 19, 2020, Pfizer and BioNTech announced they supplying an additional 100 million doses of COMIRNATY the…

On Dec. 18, 2020, the the U.S. Food and Drug Administration (FDA) approved AstraZeneca’s Tagrisso (osimertinib) as the…

On Dec. 18, 2020, the National Research Council of Canada (NRC) announced it was providing advisory services and…

On Dec. 18, 2020, the FDA issued an emergency use authorization (EUA) for the second vaccine for the…

On Dec. 18, 2020, Eli Lilly announced plans to begin a new pragmatic study of bamlanivimab (LY-CoV555) in…

On Dec. 18, 2020, Moderna announced that the European Commission (EC) had exercised its option to purchase an…
On Dec. 18, 2020, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…

On Dec. 18, 2020, Moderna announced that the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on…
On Dec. 17, 2020, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Dec. 16, 2020, Novavax announced an Advance Purchase Agreement with the government of New Zealand for the…

On Dec. 15, 2020, a months long study to determine the number of Houstonians carrying COVID-19 antibodies revealed…
On Dec. 15, 2020, AXIM Biotechnologies announced the development and patent filing for an enzyme-linked immunosorbent assay (ELISA)-based…

On Dec. 15, 2020, Inovio Pharma announced the company and a team of scientists from The Wistar Institute,…
On Dec. 14, 2020, Anixa Biosciences announced that it and partner OntoChem GmbH had verified that one of…

On Dec.14, 2020, the US Food and Drug Administration (FDA) announced they had granted Investigational New Drug (IND)…
On Dec. 14, 2020, Incyte announced that the Phase 3 RUXCOVID study evaluating the safety and efficacy of…
On Dec. 14, 2020, Moderna confirmed that the Company had concluded an agreement with the Ministry of Health…
On Dec. 12, 2020, the Advisory Committee on Immunization Practices (ACIP) issued an interim recommendation for use of…
On Dec. 11, 2020, Moderna announced that the U.S. government has exercised its option to purchase an additional…

On Dec. 10, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…
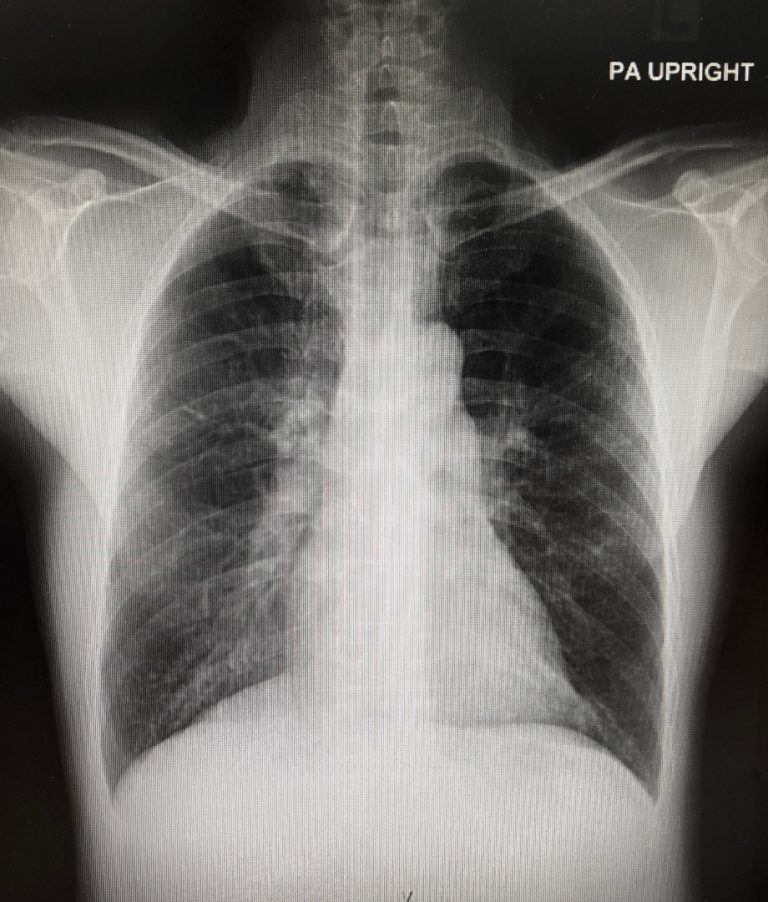
On Dec. 10, 2020, ImmunityBio announced its COVID-19 vaccine candidate protected nasal and lung airways of non-human primates…
On Dec. 10, 2020, Moderna announced that the first adolescent participants had been dosed in the Phase 2/3…

On Dec. 9, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…

On Dec. 8, 2020, Moderna announced the Swiss Federal Government had increased its confirmed order commitment from 4.5…

On Dec. 7, 2020, an universal influenza virus vaccine, which produces antibodies that target the part of the…
On Dec. 7, 2020, Moderna announced that the Canadian Government had increased its confirmed order commitment by 20…
On Dec. 7, 2020, Matinas BioPharma announced that they planned to collaborate with the National Institute of Allergy…