INOVIO dosed first subject in phase 2 segment of INNOVATE phase 2/3 trial for INO-4800 to prevent COVID-19
On Dec. 7, 2020, Inovio Pharmaceuticals announced it had dosed its first subject in a Phase 2 clinical…

On Dec. 7, 2020, Inovio Pharmaceuticals announced it had dosed its first subject in a Phase 2 clinical…

On Dec. 4, 2020, the U.S. Food and Drug Administration (FDA) authorized the first diagnostic test for at home collection…
On Dec. 4, 2020, it was reported in JAMA that high-dose influenza (commonly known as flu) vaccines are…

On Dec. 3, 2020, a new study by researchers at the University of British Columbia and BC Children’s…

On Dec. 3, 2020, Moderna announced in a letter to the editor published in the New England Journal…
On Dec. 3, 2020, AXIM Biotechnologies announced the development and patent filing for an enzyme-linked immunosorbent assay (ELISA)-based…

On Dec. 3, 2020, Inovio Pharmaceuticals announced the execution of an agreement with Kaneka Eurogentec an affiliate of…
On Dec. 2, 2020, Oxford Immunotec announces an exclusive distribution agreement with RIKEN Genesis in Japan. Under the…
On Dec. 2, 2020, BD (Becton, Dickinson) announced plans to invest approximately $1.2 billion over a 4-year period…

On Dec. 2, 2020, the University of Oregon opened the Phil and Penny Knight Campus for Accelerating Scientific…

On Nov. 30, 2020, Mateon Therapeutics announced that the India arm of ARTI-19 global study was on track…

On Nov. 30, 2020, the City of Hope reported that its investigational vaccine produced strong protective immunity against…

On Nov. 30, 2020, Moderna announced that the primary efficacy analysis of the Phase 3 study of mRNA-1273…

On Nov. 30, 2020, Innovation Pharmaceuticals announced additional independent preliminary laboratory research suggests Brilacidin, the Company’s flagship defensin…

On Nov. 30, 2020, Novavax announced that two of the three planned late-stage efficacy trials for NVX-CoV2373 sponsored…

On Nov. 30, 2020, Lonza announced $10 million investment in its Bend, Oregon facility. Originally known as Bend…
On Nov. 29, 2020, Moderna announced a supply agreement with the United Kingdom (UK) government for an additional…

On Nov. 25, 2020, RedHill Biopharma announced partnerships with two leading, U.S.-based manufacturers for large-scale manufacturing of opaganib….
On Nov. 25, 2020, Moderna announced that the European Commission (EC) has approved an agreement to secure 80…

On Nov. 25, 2020, BioNTech and InstaDeep announced a multi-year strategic collaboration aimed at applying the latest advances…
On Nov. 24, 2020, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…

On Nov. 24, 2020, RELIEF THERAPEUTICS announced that more than 175 patients with Critical COVID-19 and Respiratory Failure…
On Nov. 24, 2020, Northwestern University researchers announced they had developed a new artificial intelligence (A.I.) platform that…

On Nov. 24, 2020, Todos Medical announced that it has completed the installation of Tecan’ laboratory automated equipment…

On Nov. 23, 2020, the U.S. Food and Drug Administration (FDA) expanded the approved indication for Genentech’s Xofluza…

On Nov. 23, 2020, CytoDyn announced it had reached enrollment of 293 patients in its Phase 3 trial…

On Nov. 20, 2020, the ‘European Food Safety Authority reported that within the past month more than 300…
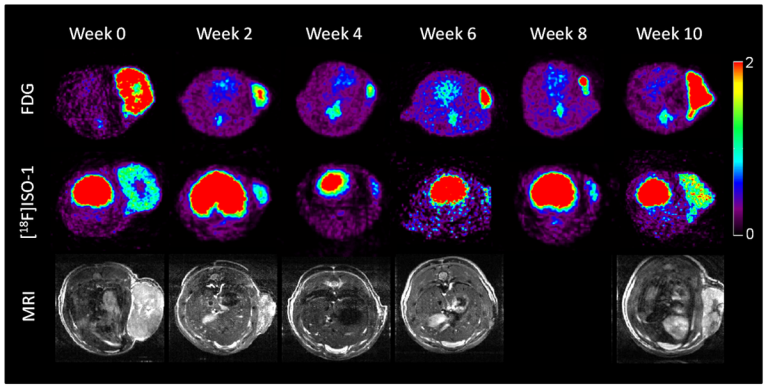
On Nov. 19, 2020, in a comprehensive analysis of patients with cancer who had exceptional responses to therapy,…
On Nov. 19, 2020, University of Oxford researchers announced that research into the HIV-1 virus had shed light…
On Nov. 19, 2020, Novartis announced that it had entered into an exclusive worldwide license and collaboration agreement…