Mateon announced peer reviewed publication on its R&D effort against COVID-19
On Apr. 30, 2020, Mateon Therapeutics reported several peer-reviewed publications derived from its R&D effort against COVID-19. Published…

On Apr. 30, 2020, Mateon Therapeutics reported several peer-reviewed publications derived from its R&D effort against COVID-19. Published…
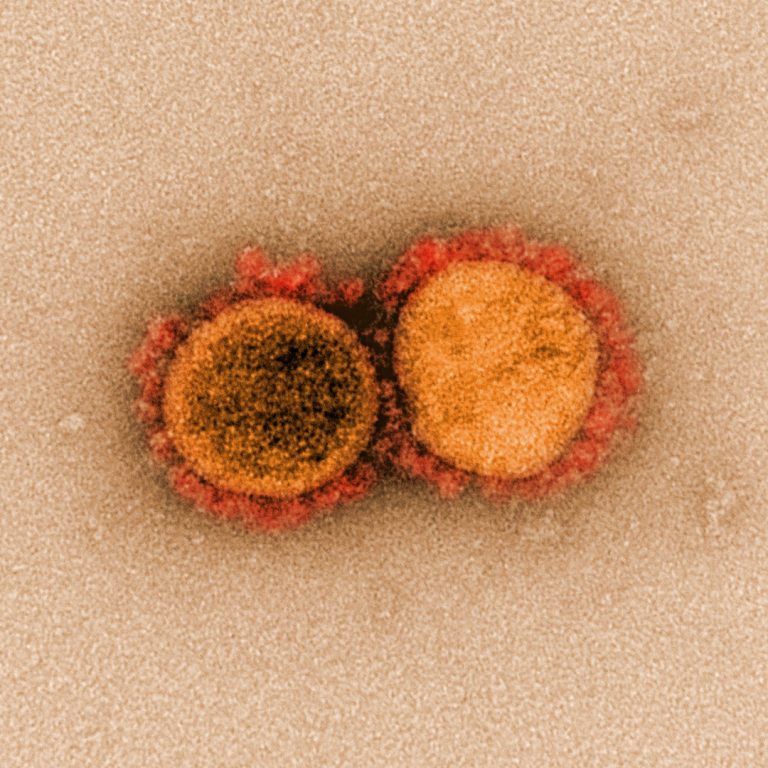
On Apr. 30, 2020, an international team of more than 120 scientists has detailed the impact of 75…
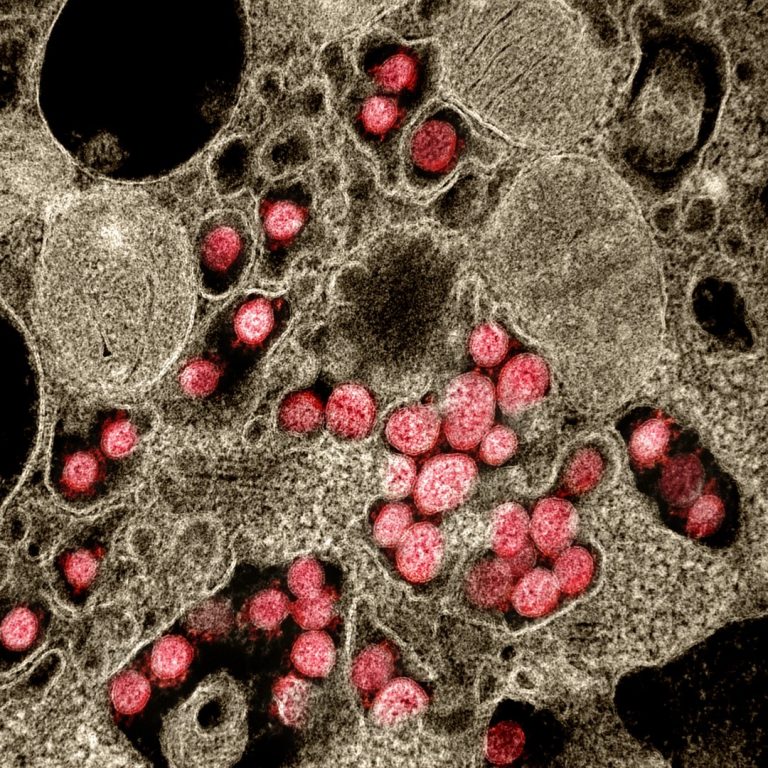
On Apr. 30, 2020, BioSig Technologies announced that an article titled, “The IMPDH inhibitor merimepodib provided in combination…
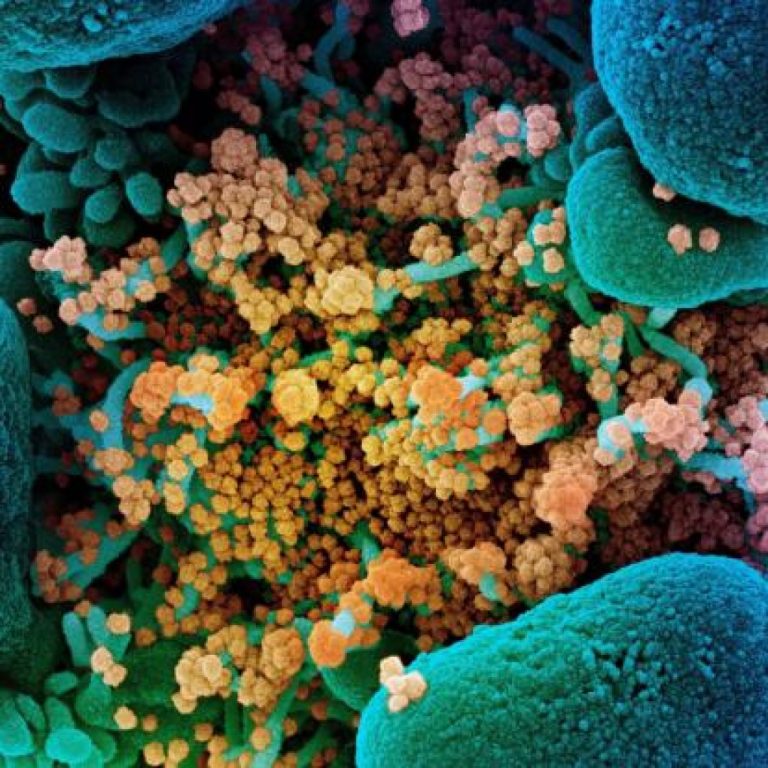
On Apr. 30, 2020, Emory University researchers are taking part in a multi-site study across the U.S. to…
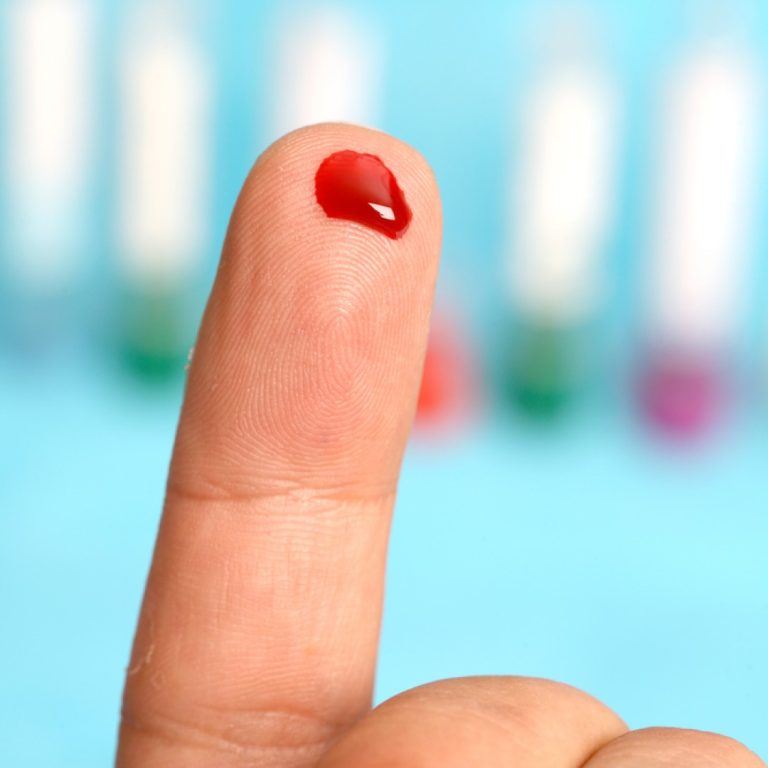
On Apr. 30, 2020, InBios announced that it had been awarded a contract from the Biomedical Advanced Research…
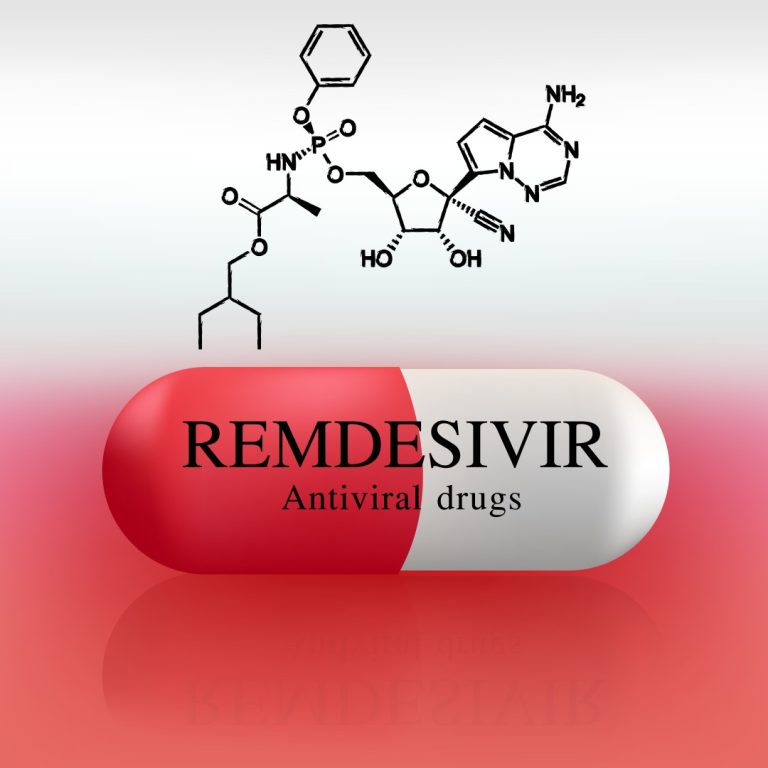
On Apr. 29, 2020, the National Institutes of Health (NIH) announced that hospitalized patients with advanced COVID-19 and…
On Apr. 29, 2020, Scripps researchers announced they had developed a method for examining the machinery of HIV…

On Apr. 29, 2020, Emory University played a leading role in the government-sponsored clinical trial of the COVID-19…
On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine and NYU Langone…

On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine announced that they…
On Apr. 29, 2020, Windtree Therapeutics announced it has enrolled the first patient into its AEROSURF phase 2b…
On Apr. 28, 2020, Entos Pharmaceuticals announced a collaboration with EpiVax. This partnership combined the Entos Fusogenix DNA…

On Apr. 28, 2020, Illumina announced that the African Union Commission, on behalf of the Africa Center for…
On Apr. 28, 2020, Secarna Pharmaceuticals announced the company has entered into a cooperation with the First Affiliated…

On Apr. 28, 2020, Sarepta Therapeutics announced the Company and the USAMRIID, the Department of Defense’s lead laboratory…
On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine announced that a…

On Apr. 28, 2020, INOVIO and GeneOne Life Science announced interim data through week 16 from a Phase…
On Apr. 28, 2020, researchers at the Great Lakes Bioenergy Research Center at the University of Wisconsin-Madison have…

On Apr. 27, 2020, RedHill Biopharma provided an additional update on the compassionate use program with its investigational…

On Apr. 27, 2020, Mateon Therapeutics announced announced it has submitted an Investigational New Drug application to the…

On Apr. 27, 2020, Yield10 Bioscience announced it has obtained a positive response from USDA-APHIS’s Biotechnology Regulatory Services…

On Apr. 27, 2020, Neurocrine Biosciences announced the FDA approved once-daily oral ONGENTYS (opicapone) 25 mg and 50…

On Apr. 27, 2020, ARTES Biotechnology, the German-based biotech company specializing in process development for recombinant vaccines, entered…
On Apr. 27, 2020, Moderna announced it had submitted an Investigational New Drug application to the U.S. Food…
On Apr. 27, 2020, BioAegis Therapeutics announced it had published gene expression data in animal studies where recombinant…

On Apr. 27, 2020, Regeneron and Sanofi announced preliminary results from the Phase 2 portion of an ongoing…

On Apr. 27, 2020, Regeneron and Sanofi announced the primary endpoint of overall survival was met in a…

On Apr. 27, 2020, iBio announced it has joined the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL),…

On Apr. 27, 2020, Ichor announced that its TriGrid Delivery System is being utilized for delivery in non-human…

On Apr. 27, 2020, Merck and the Institute for Systems Biology announced a new research collaboration to investigate…