University of Minnesota researchers orchestrated state’s fastest on-site testing for COVID-19
On Mar. 31, 2020, the University of Minnesota announced that the Medical School transformed two research buildings to…
On Mar. 31, 2020, the University of Minnesota announced that the Medical School transformed two research buildings to…

On Mar. 31, 2020, a team led by Pawel Kalinski, MD, PhD, of Roswell Park Comprehensive Cancer Center…

On Mar. 31, 2020, Luminex announced it had received $642,450 in funding from the Biomedical Advanced Research and…
On Mar. 30, 2020, the partners in the COVID-19 Therapeutics Accelerator announced grants of $20 million to three…

On Mar. 30, 2020, the Translational Genomics Research Institute (TGen) announced that it had joined the world-wide effort…
On Mar. 30, 2020, the La Jolla Institute for Immunology (LJI) announced it had been awarded a $1.73…
On Mar. 30, 2020, Altimmune announced that it was launching a collaboration with the University of Alabama at…

On Mar. 28, 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to…

On Mar. 27, 2020, Medtronic announced its Medtronic Care Management Services (MCMS) business had launched two new solutions…
On Mar. 27, 2020, in response to the crisis caused by the COVID-19 virus in California and around…

On Mar. 27, 2020, Humanigen announced that the company had submitted an initial protocol synopsis to U.S. Food…

On Mar. 27, 2020, Emory’s Vaccine and Treatment Evaluation Unit (VTEU) announced participation in a clinical trial testing…

On Mar. 26, 2020, Thermo Fisher Scientific announced it had received the CE mark in the European Union…
On Mar. 26, 2020, Amyris announced that it had completed initial testing of a leading vaccine adjuvant. In…
On Mar. 26, 2020, physician-scientists at four University of California Health medical centers — UC San Diego Health,…
On Mar. 26, 2020, working in partnership with the Commonwealth of Massachusetts, Massachusetts State Public Health Laboratory, and…
On Mar. 26, 2020, Henry Schein announced the availability of an antibody rapid blood test, known as Standard…

On Mar. 25, 2020, NASA’s Jet Propulsion Laboratory (JPL) in Southern California announced that after receiving more than…
On Mar. 25, 2020, the National Library of Medicine (NLM), part of the National Institutes of Health, announced…

On Mar. 25, 2020, a consortium of life sciences companies announced an important collaboration to accelerate the development,…
On Mar. 25, 2020, Mateon Therapeutics announced an update on its rapid antiviral response program targeting coronaviruses, initially…

On Mar. 25, 2020, Xencor announced a technology license agreement with Vir Biotech in which Vir will have…

On Mar. 25, 2020, the Jackson Laboratory (JAX) announced it was working to ensure that the worldwide research…

On Mar. 25, 2020, the Perelman School of Medicine at the University of Pennsylvania announced it had established…

On Mar. 24, 2020, Partner Therapeutics that Leukine (sargramostim, rhu-GM-CSF) is being assessed in the SARPAC trial (sargramostim…

On Mar. 24, 2020, T2 Biosystems announced it had entered into a worldwide licensing agreement for a rapid…
On Mar. 24, 2020, Novavax announced positive top-line results of its pivotal Phase 3 clinical trial of NanoFlu,…
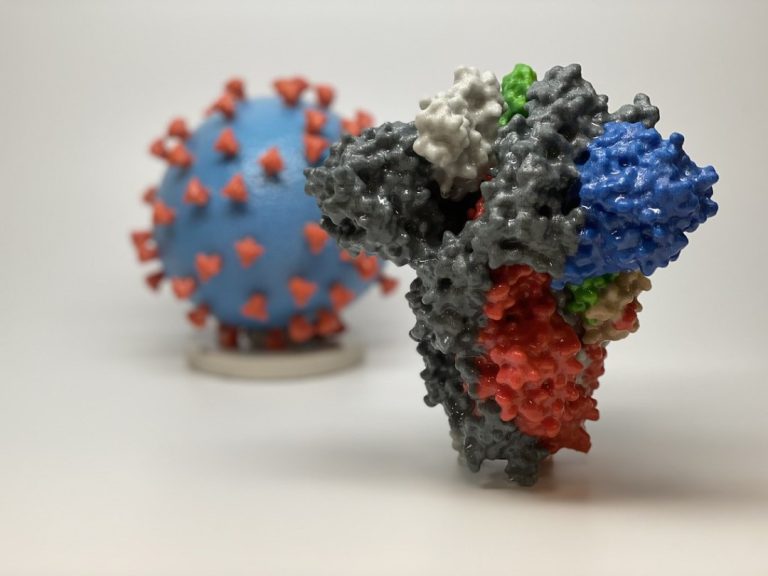
On Mar. 24, 2020, to help in educating the public and in the hope of better conveying the…
On Mar. 24, 2020, the University of Alberta (U of A) announced that a Public health study was…

On Mar. 24, 2020, bioMerieux announced that BioFire Diagnostics, its subsidiary specialized in syndromic infectious disease testing, had…