Therapeutic Goods Administration of Australia authorized Moderna’s Covid-19 vaccine in children (6-11 Years)
On Feb. 16, 2022, Moderna announced that the Therapeutic Goods Administration in Australia had granted provisional registration for…
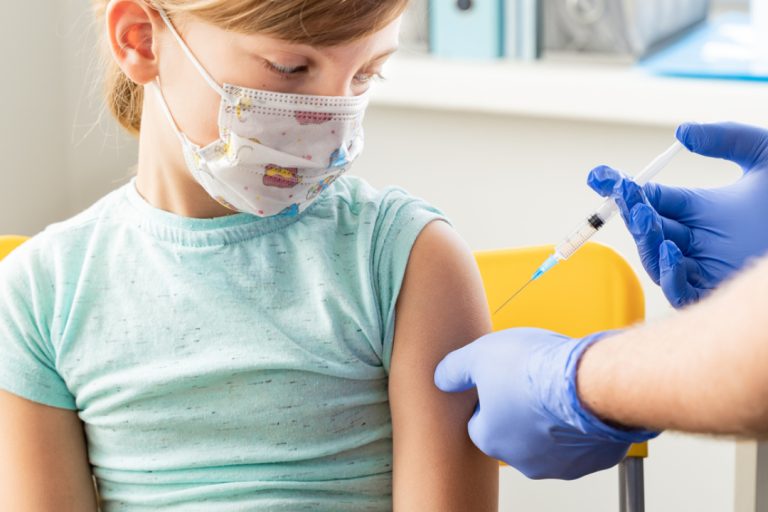
On Feb. 16, 2022, Moderna announced that the Therapeutic Goods Administration in Australia had granted provisional registration for…

On Feb. 14, 2022, Novavax announced that the Singapore Health Sciences Authority (HSA) had issued interim authorization for…

On Feb. 14, 2022, Novavax announced its submission to Swissmedic, the Swiss Agency for Therapeutic Products, for conditional…
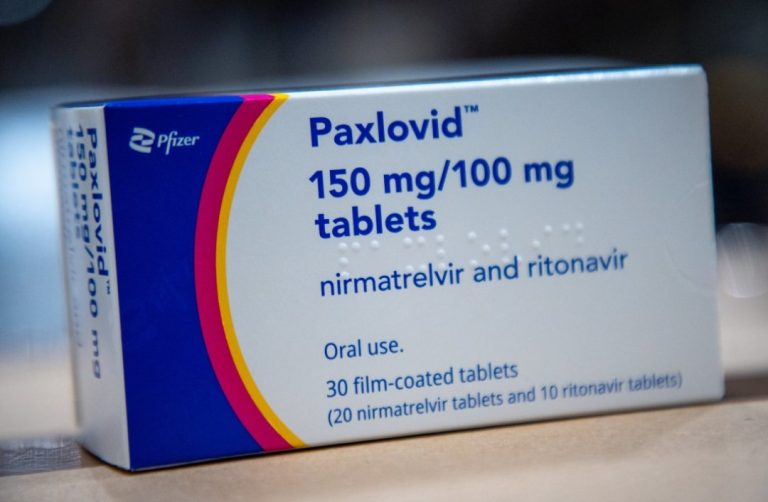
On Feb. 12, 2022, China’s medical products regulator announced that it had given conditional approval for Pfizer’s COVID-19…

On Feb. 11, 2022, Pfizer and BioNTech announced plans to extend their rolling submission to the U.S. Food…

On Feb. 11, 2022, Roche announced that Actemraᆴ/RoActemraᆴ (tocilizumab) intravenous (IV) had been granted World Health Organization (WHO)…

On Feb. 11, 2022, the WHO announced the addition of tocilizumab, a monoclonal antibody, to its list of…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…
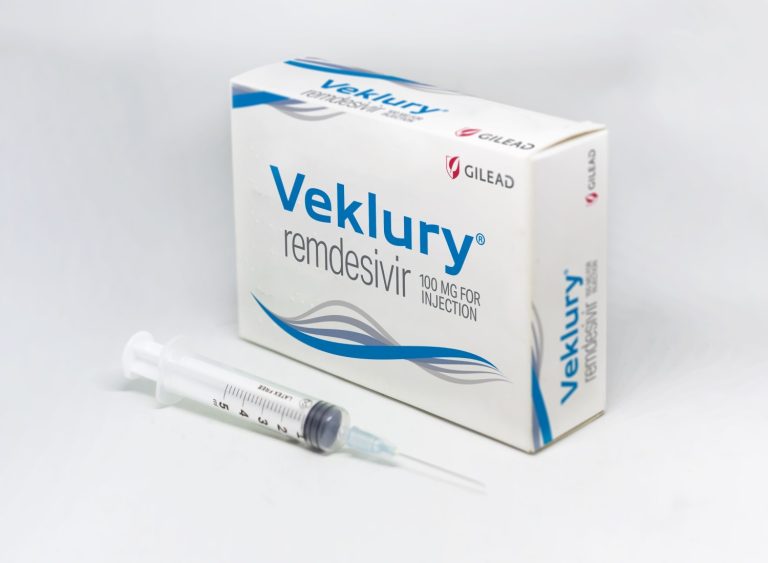
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 10, 2022, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, achieved its primary effectiveness…
On Feb. 8, 2022, Moderna announced a new supply agreement with the government of Colombia for 10.8 million…

On Feb. 8, 2022, Merck and Ridgeback Biotherapeutics announced that a total of 3.1 million courses of molnupiravir,…

On Feb. 8, 2022, a research team led at Boston University School of Public Health reported on links…

On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…
On Feb. 7, 2022, researchers at Washington University School of Medicine in St. Louis announced an analysis of…

On Feb. 4, 2022, a new ᆪ1.6m collaborative project was launched to rapidly identify new treatments for COVID-19….

On Feb. 4, 2022, pet hamsters probably carried the Delta variant of SARS-CoV-2 into Hong Kong and sparked…

On Feb. 4, 2022, the Advisory Committee on Immunization Practices (ACIP) issued a standard recommendation for use of…

On Feb. 3, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency had granted conditional marketing…

On Feb. 3, 2022, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected Omicron COVID-19 subvariants BA.1, BA.2, and…

On Feb. 3, 2022, Novavax announced that New Zealand’s Medsafe had been granted provisional approval of NVX-CoV2373, Novavax’…
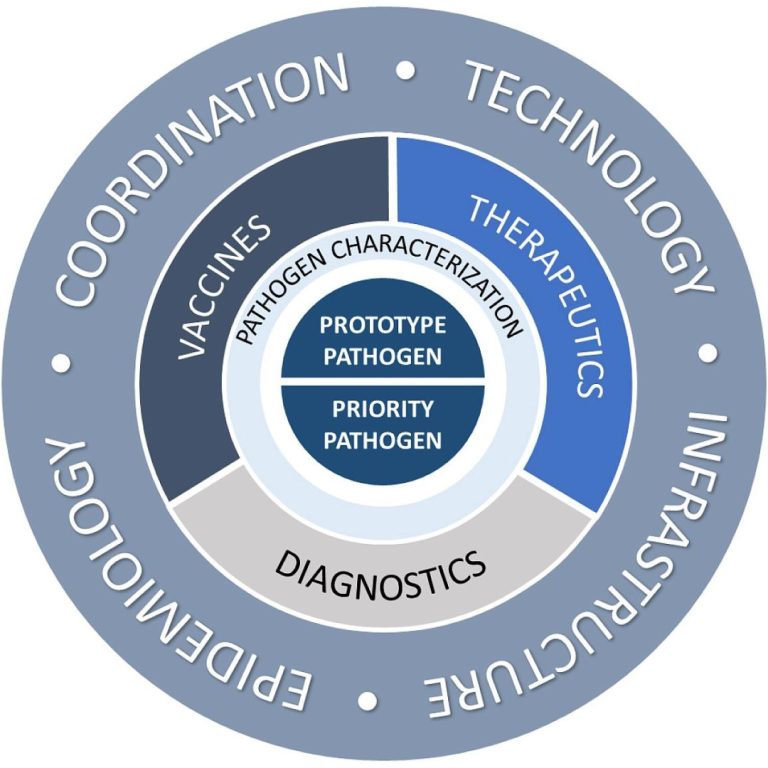
On Feb. 2, 2022, NIAID scientists announced the new Pandemic Preparedness Plan aimed to support critical basic and…

On Feb. 1, 2022, Pfizer and BioNTech announced that following a request from the U.S. Food and Drug…

On Jan. 31, 2022, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the Biologics License…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…

On Jan. 31, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests had been authorized by the…

On Jan. 31, 2022, Veru announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track…

On Jan. 28, 2022, Novavax and Israel’s Ministry of Health announced an agreement for the purchase of NVX-CoV2373,…

On Jan. 28, 2022, the WHO announced that COVID-19 information had reached 1,292,209 people through Viamoメs 3-2-1 Platform…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…