Moderna announced supply agreement with the UK for additional 60 million doses of COVID-19 vaccine in 2022 and 2023
On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…

On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…
On Dec. 1, 2021, the California and San Francisco Departments of Public Health confirmed that a recent case…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…
On Nov. 30, 2021, LabCorp announced that it had begun offering observed self-collection for COVID-19 PCR testing in…

On Nov. 29, 2021, Thermo Fisher Scientific confirmed that its polymerase chain reaction (PCR) TaqPath COVID-19 Combo Kit…

On Nov. 29, 2021, Hologic announced that its three SARS-CoV-2 tests all detected the recently emerged Omicron variant…
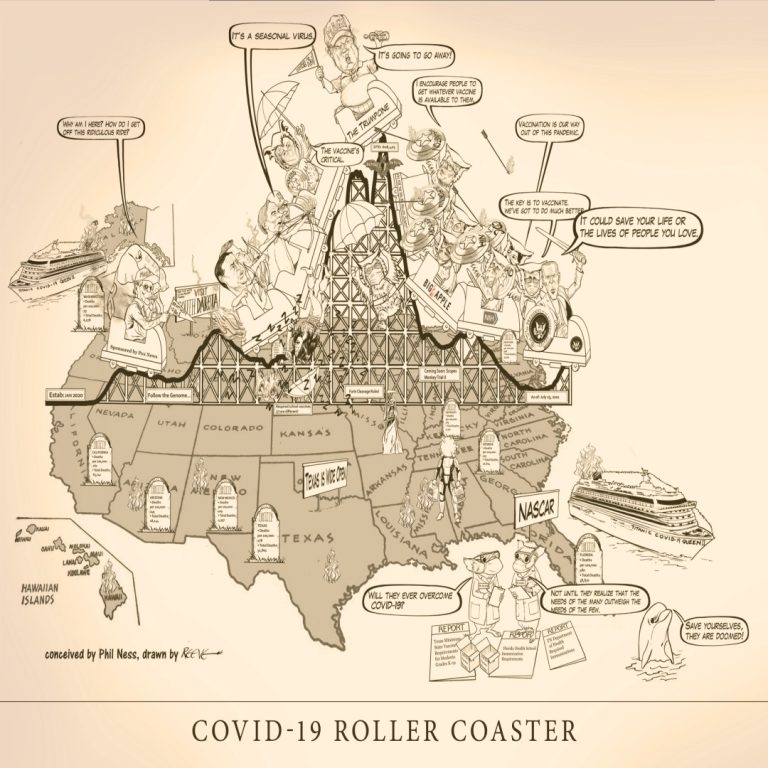
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…

On Nov. 25, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Nov. 24, 2021, Novavax announced its submission to the Singapore Health Sciences Authority for interim authorization of…

On Nov. 24, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Nov. 23, 2021, the World Health Organization’s COVID-19 Technology Access Pool and the Medicines Patent Pool finalized…
On Nov. 22, 2021, Tonix Pharmaceuticals announced the publication of ‘Sangivamycin is highly effective against SARS-CoV-2 in vitro…
On Nov. 22, 2021, Pfizer announced topline results from a longer-term analysis of the safety and efficacy of…

On Nov. 22, 2021, the U.S. Food and Drug Administration authorized another over-the-counter (OTC) COVID-19 antigen test. The…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…

On Nov. 19, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, Novavax announced that the European Medicines Agency (EMA) had begun its evaluation of an…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 17, 2021, GlaxoSmithKline and Vir Biotechnology announced U.S. government contracts totalling approximately $1 billion (USD) to…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 16, 2021, Pfizer and the Medicines Patent Pool (MPP), a United Nations-backed public health organization working…

On Nov. 16, 2021, an agreement was announced that enabled the European Union and European Economic Area countries…

On Nov. 15, 2021, Novavax and SK bioscience announced submission of a Biologics License Application (BLA) for Novavax’…
On Nov. 15, 2021, Moderna confirmed that Health Canada had authorized the use of a booster dose of…

On Nov. 15, 2021, a large, long-term study of the impacts of COVID-19 on children announced it had…

On Nov. 15, 2021, AstraZeneca reported that 2 billion doses of the COVID-19 vaccine had been released for…

On Nov. 12, 2021, Regeneron announced that the European Commission (EC) had approved the casirivimab and imdevimab antibody…