The FDA approved first therapy for rare disease that causes low phosphate blood levels, bone softening
On Jun. 18, 2020, the U.S. Food and Drug Administration (FDA) approved Ultragenyx Pharmaceutical ‘s Crysvita (burosumab-twza) injection…

On Jun. 18, 2020, the U.S. Food and Drug Administration (FDA) approved Ultragenyx Pharmaceutical ‘s Crysvita (burosumab-twza) injection…

On Jun. 17, 2020, Aclaris Therapeutics announced that the FDA had allowed an investigational new drug application to…

On Jun. 16, 2020, the FDA revoked the emergency use authorization (EUA) of the Chembio Diagnostic System DPP…
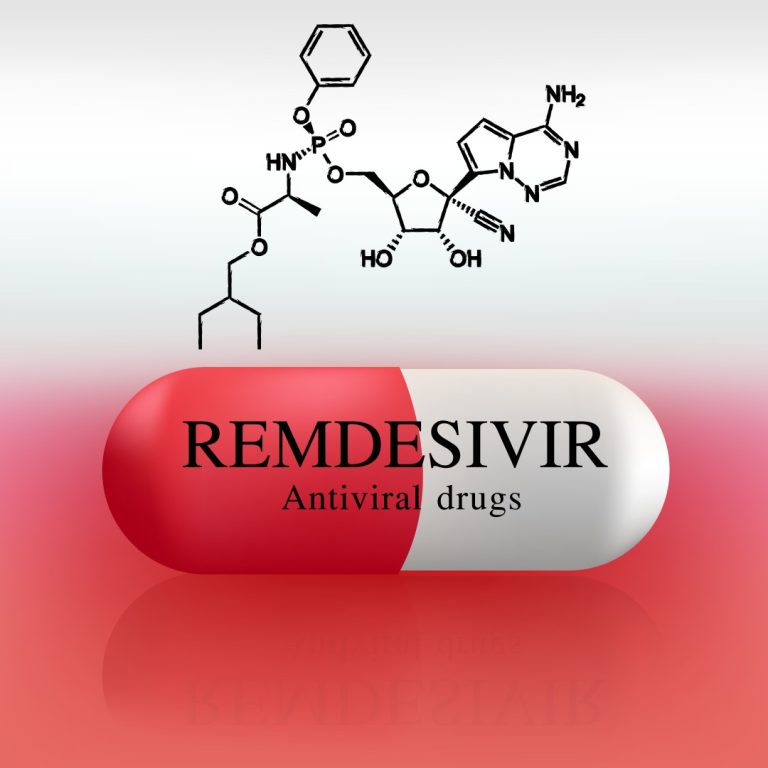
On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) warned health care providers about a newly…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) revoked the emergency use authorization (EUA) that…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) permitted marketing of the first game-based digital…

On Jun. 15, 2020, SIGA Technologies announced the U.S. Department of Defense (DoD) increased research and development funding…

On Jun. 12, 2020, the FDA announced it had approved an expanded indication for Merck’s GARDASIL9 for the…

On Jun. 12, 2020, ViiV Healthcare, a GSK company, with Pfizer and Shionogi Limited, announced the FDA had…

On Jun. 11, 2020, InBios announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Jun. 11, 2020, Bristol-Myers Squibb announced that Opdivo (nivolumab) was approved by the FDA for the treatment…

On Jun. 11, 2020, the FDA approved Viela Bio’s Uplizna (inebilizumab-cdon) injection for intravenous use for the treatment…

On Jun. 11, 2020, Moderna announced progress on late-stage development of mRNA-1273, the Company’s mRNA vaccine candidate against…

On Jun. 10, 2020, the FDA issued an emergency use authorization (EUA) to Illumina for the first COVID-19…
On Jun. 9, 2020, Illumina announced the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization…

On Jun. 9, 2020, Quidel announced it had received an amended Emergency Use Authorization (EUA) from the FDA,…

On Jun. 9, 2020, Immunic announced receipt of regulatory allowance from the FDA to initiate its phase 2,…

On Jun. 8, 2020, OraSure Technologies announced that Phosphorus Diagnostics, a leader in diagnostic and bioinformatic solutions for…
On Jun. 4, 2020, the FDA approved Merck’s Recarbrio (a combination of imipenem-cilastatin and relebactam) to treat hospital-acquired…

On Jun. 2, 2020, Agenus announced the FDA’s clearance of AgenTus’ IND application for an allogeneic iNKT therapy….

On Jun. 1, 2020, the FDA issued an emergency use authorization (EUA) for Impella RP to include patients…

On Jun. 1, 2020, Altimmune announced the FDA had authorized the Company to proceed with a clinical trial…

On Jun. 1, 2020, OPKO Health announced the FDA had authorized OPKO to undertake a Phase 2 trial…

On May 29, 2020, the FDA took steps to further support the development of COVID-19 tests for at-home…
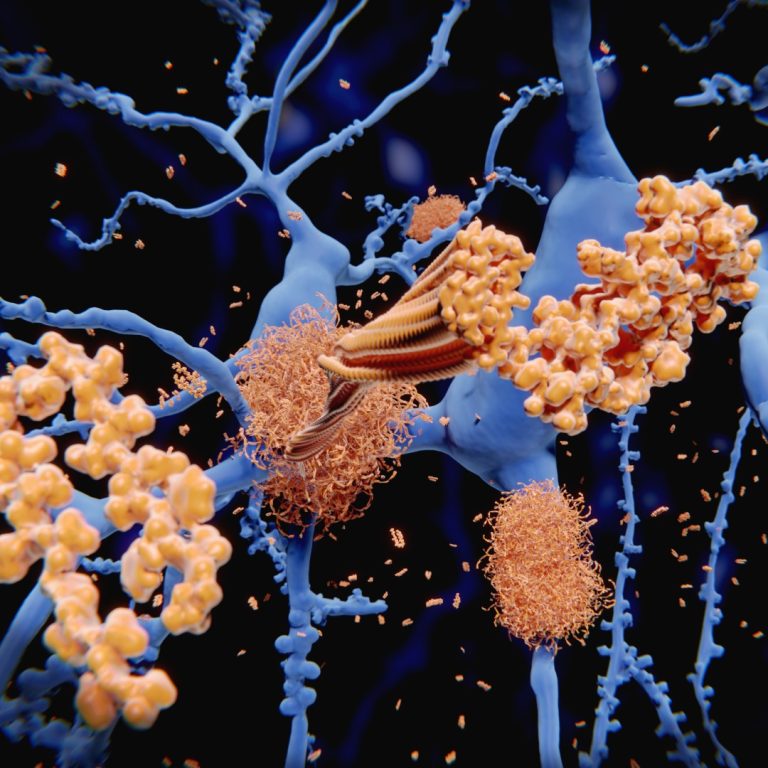
On May 28, 2020, the FDA approved Avid Radiopharmaceuticals’ Tauvid (flortaucipir F18) for intravenous injection, the first drug…

On May 28, 2020, Quest Diagnostics announced it had received emergency use authorization (EUA) from the FDA for…

On May 26, 2020, the FDA approved Amivas’ artesunate for injection to treat severe malaria in adult and…

On May 26, 2020, Royal Philips announced it had received 510(k) clearance from the FDA for its wearable…

On May 26, 2020, the FDA provided approval to Amivas for its artesunate for injection to treat severe…

On May 26, 2020, Meridian Bioscience announced that its SARS-CoV-2 antigens and related reagents are part of assays…