Diffusion Pharma announced FDA Accelerated Review of TSC clinical development plan to treat COVID-19 patients with ARDS
On May 5, 2020, Diffusion Pharmaceuticals announced the FDA accelerated its review of the Companyメs clinical development plan…

On May 5, 2020, Diffusion Pharmaceuticals announced the FDA accelerated its review of the Companyメs clinical development plan…

On May 4, 2020, bioMerieux announced that BioFire Diagnostics, its subsidiary specialized in syndromic infectious disease testing, has…

On May 4, 2020, Vir Biotech and Alnylam Pharma announced the selection of a development candidate for VIR-2703,…

On May 4, 2020, Cerus announced FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative…

On May 4, 2020, DNA Genotek, a subsidiary of OraSure Technologies, announced that its ORAcollect RNA kit (OR-100)…

On May 2, 2020, Roche announced that the FDA has issued an Emergency Use Authorization for its new…
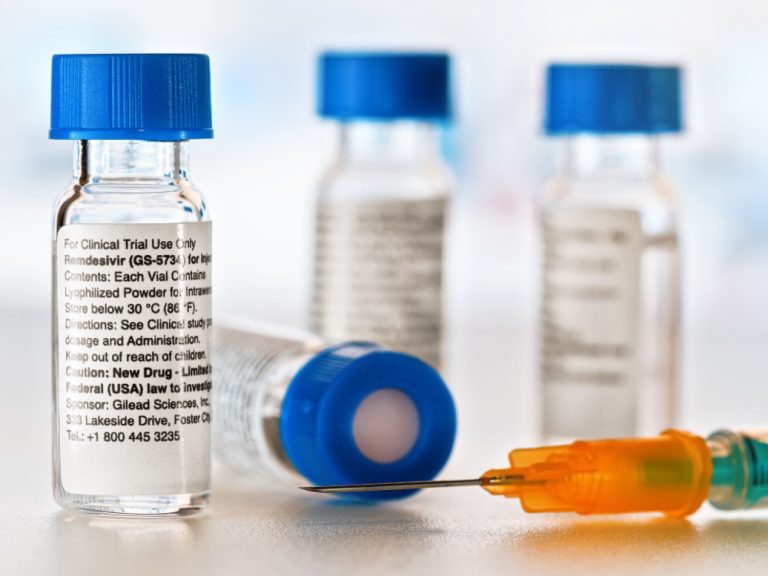
On May 1, 2020, Gilead announced the FDA granted emergency use authorization (EUA) for the investigational antiviral remdesivir…

On May 1, 2020, the FDA issued an Emergency Use Authorization (EUA) for the investigational antiviral drug remdesivir…

On May 1, 2020, Janssen Pharma, a Johnson & Johnson company, announced the U.S. Food and Drug Administration…

On Apr. 30, 2020, the FDA included, under the ventilator emergency use authorization (EUA), a ventilator developed by…

On Apr. 28, 2020, Chimerix announced it had received clearance from the FDA for a rolling submission of…

On Apr. 27, 2020, Mateon Therapeutics announced announced it has submitted an Investigational New Drug application to the…

On Apr. 27, 2020, Neurocrine Biosciences announced the FDA approved once-daily oral ONGENTYS (opicapone) 25 mg and 50…
On Apr. 27, 2020, Moderna announced it had submitted an Investigational New Drug application to the U.S. Food…

On Apr. 27, 2020, Diffusion Pharmaceuticals announced the pre-IND submission to the FDA of a planned clinical program…

On Apr. 24, 2020, the U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication regarding known…

On Apr. 24, 2020, BioSig Technologies announced that subsidiary ViralClear had submitted an Investigational New Drug (IND) application…

On Apr. 23, 2020, Baxter announced it had received emergency use authorization (EUA) from the FDA for the…

On Apr. 22, 2020, the FDA issued an emergency use authorization (EUA) for the Philips Medizin Systeme Boeblingen…

On Apr. 23, 2020, Caladrius Biosciences announced the FDA has authorized its investigational new drug application for the…
On Apr. 22, 2020, LivaNova announced that several of its cardiopulmonary products are now permitted to be used…

On Apr. 22, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy),…
On Apr. 22, 2020, Quidel announced it has applied for an Emergency Use Authorization (EUA) for the Lyraᆴ…

On Apr. 22, 2020, ALung Technologies announced the U.S. Food and Drug Administration (FDA) granted the Company Emergency…
On Apr. 21, 2020, the Janssen Pharmaceutical Companies of Johnson & Johnson announced U.S. Food and Drug Administration…

On Apr. 21, 2020, aTyr Pharma announced the FDA has accepted its Investigational New Drug application to evaluate…
On Apr. 21, 2020, BioSig Technologies announced that subsidiary ViralClear Pharmaceuticals, had submitted an application for Vicromax(tm) (merimepodib,…

On Apr. 21, 2020, LabCorpᆴ announced it has received an Emergency Use Authorization (EUA) from the FDA. The…

On Apr. 21, 2020, Todos Medical announced that Gnomegen has received Emergency Use Authorization from the FDA for…

On Apr. 20, 2020, Novartis announced it had reached an agreement with the U.S. Food and Drug Administration…