FDA approved Epclusa (Sofosbuvir/Velpatasvir) for children with Chronic Hepatitis C infection
On Mar. 19, 2020, Gilead Sciences announced the U.S. Food and Drug Administration (FDA) had approved a supplemental…
On Mar. 19, 2020, Gilead Sciences announced the U.S. Food and Drug Administration (FDA) had approved a supplemental…

On Mar. 18, 2020, Abbott announced that the FDA had issued Emergency Use Authorization (EUA) for the company’s…

On Mar. 18, 2020, Mateon Therapeutics reported significant progress in deploying its phase 3 clinical asset, OT-101, against…
On Mar. 18, 2020, Eli Lilly announced its scientists were partnering with the Indiana State Department of Health…

On Mar. 17, 2020, Quidel Corp. announced it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Mar. 17, 2020, BioMedomics announced it will be able to distribute their COVID-19 IgM-IgG Antibody Rapid Test…
On Mar. 17, 2020, Todos Medical announced a non-exclusive distribution agreement with 3D Biomedicine Science & Technology, a…

On Mar. 16, 2020, Aimmune Therapeutics announced that the first patients in the U.S. were being treated with…

On Mar. 16, 2020, Hologic announced the U.S. Food and Drug Administration (FDA) had granted Emergency Use Authorization…

On Mar. 16, 2020, BD (Becton, Dickinson and Company) announced the companies had submitted Emergency Use Authorization requests…

On Mar. 13, 2020, Roche announced the FDA has issued an Emergency Use Authorization for the cobasᆴ SARS-CoV-2…

On Mar. 13, 2020, Thermo Fisher Scientific announced the FDA has issued an emergency use authorization (EUA) for…

On Mar. 9, 2020, the FDA approved Boehringer Ingelheim’s Ofev (nintedanib) oral capsules to treat patients with chronic…

On Mar. 6, 2020, the FDA approved Isturisa (osilodrostat) oral tablets for adults with Cushing’s disease who either…

On Mar. 4, 2020, the UW Medicine Clinical Virology Lab announced it had received U.S. Food and Drug…
On Mar. 2, 2020, Mateon Therapeutics reported the company had been evaluating its therapeutic and AI platforms to…

On Feb. 29, 2020, the University of Washington (UW) Medicine Clinical Virology Lab announced it had received U.S….

On Feb. 28, 2020, the FDA approved an application for the first generic of Daraprim (pyrimethamine) tablets for…

On Feb. 24, 2020, Innovation Pharmaannounced the Company submitted a Material Transfer Agreement (MTA) with a leading U.S.-based…

On Feb. 22, 2020, a federal judge from the Southern District of New York ruled drug companies, device…

On Feb. 21, 2020, the U.S. Food and Drug Administration (FDA) approved NEXLETOL (bempedoic acid) tablet, an oral,…

On Feb. 21, 2020, the U.S. Food and Drug Administration (FDA) authorized marketing of the first test to…

On Feb. 18, 2020, Innovation Pharma announced the Company was exploring its lead defensin mimetic drug candidate, Brilacidin,…
On Jan. 14, 2020, Valneva announced that the U.S. Department of Defense (DoD) had exercised an option to…

On Jan. 8, 2020, the U.S. Food and Drug Administration (FDA) approved patient-specific airway stents developed by Cleveland…

On Dec. 20, 2019, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Daiichi Sankyo’s Enhertu…

On Dec. 18, 2019, Seagen and Astellas Pharma announced the U.S. Food and Drug Administration (FDA) had granted…

On Dec. 17, 2019, the University of Alabama at Birmingham Comprehensive Cancer Center (UAB) announced that Gregory Friedman,…
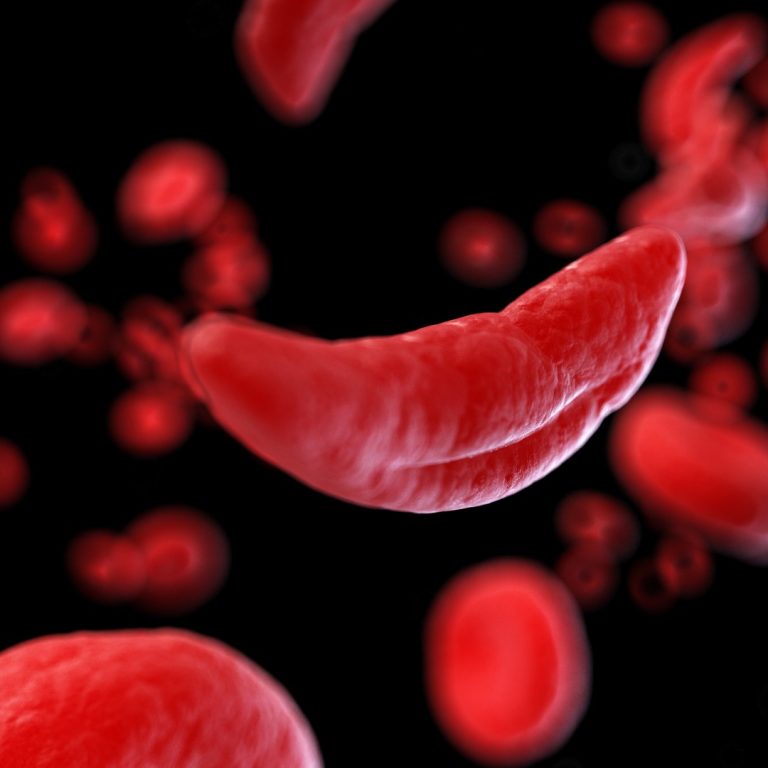
On Dec. 2, 2019, the U.S. Food and Drug Administration (FDA) announced it had approved the drug crizanlizumab…
On Nov. 22, 2019, the Centers for Disease Control and Prevention”s (CDC) Advisory Committee on Immunization Practices (ACIP)…