Labcorp received FDA Emergency Use Authorization for Mpox PCR test home collection kit
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…
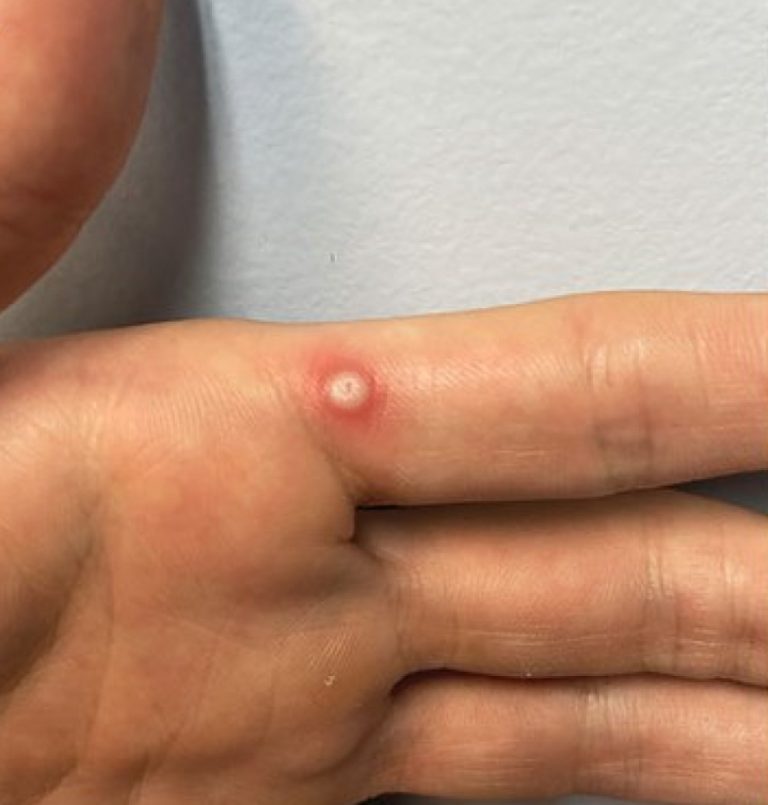
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Apr. 1, 2024, Otsuka Pharmaceutica and Click Therapeutics announced that the U.S. Food and Drug Administration (FDA)…
On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…

On Mar. 22, 2024, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Pemgarda…

On Mar. 21, 2024, the U.S. Food and Drug Administration (FDA) approved Italfarmaco’s Duvyzat (givinostat) oral medication for…

On Mar. 18, 2024, the U.S. Food and Drug Administration (FDA) approved Orchard Therapeutics’ Lenmeldy (atidarsagene autotemcel), the…
On Mar. 14, 2024, the U.S. Food and Drug Administration (FDA) approved Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) for the…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…

On Mar. 5, 2024, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC)…

On Mar. 4, 2024, Hugel America announced it received U.S. Food and Drug Administration (FDA) approval on its…

On Feb. 28, 2024, the U.S. Food and Drug Administration (FDA) announced that grease-proofing materials containing per- and…

On Feb. 16. 2024, Roche announced today that the U.S. Food and Drug Administration (FDA) has approved Xolair…

On Feb. 14, 2024, the U.S. Food and Drug Administration (FDA) approved Eicos Sciences’ Aurlumyn (iloprost) injection to…

On Jan. 16, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved CRISPR Therapeutics’ CASGEVY…

On Jan. 15, 2024, CSL announced that Swissmedic had authorised HEMGENIX® (etranacogene dezaparvovec), the first and currently only gene…

On Jan. 5, 2024, the U.S. Food and Drug Administration (FDA) authorized Florida’s Agency for Health Care Administration’s…

On Dec. 19, 2023, the U.S. Food and Drug Administration approved the first test that uses DNA in…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…
On Dec. 5, 2023, Novartis announced that the U.S. Food and Drug Administration (FDA) had approved Fabhaltaᆴ (iptacopan)…
On Nov. 28, 2023, Emergent BioSolutions announced that the Biomedical Advanced Research and Development Authority (BARDA) within the…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…

On Nov. 17, 2023, Masimo announced that the Masimo W1 medical watch has received FDA 510(k) clearance for…

On Nov. 15, 2023, the U.S. Food and Drug Administration granted marketing authorization to LetsGetChecked for the Simple…
On Nov. 9, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC)…
On Nov. 2, 2023, Abbott announced that it had received U.S. Food and Drug Administration (FDA) approval for…

On Oct. 31, 2023, Novartis announced that the US Food and Drug Administration (FDA) had approved Cosentyxᆴ (secukinumab)…

On Oct. 24, 2023, the U.S. Food and Drug Administration approved Servier Pharmaceuticals’ Tibsovo (ivosidenib) for the treatment…