FDA approved new therapy for rare form of blood cancers called myelodysplastic syndromes
On Oct. 24, 2023, the U.S. Food and Drug Administration approved Servier Pharmaceuticals’ Tibsovo (ivosidenib) for the treatment…

On Oct. 24, 2023, the U.S. Food and Drug Administration approved Servier Pharmaceuticals’ Tibsovo (ivosidenib) for the treatment…
On Oct. 20, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PENBRAYA (meningococcal…

On Oct. 3, 2023, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of…
On Oct. 3, 2023, Novavax announced that it’s COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601) had received Emergency Use…

On Sept. 29, 2023, the U.S. Food and Drug Administration (FDA) granted Invitae de novo marketing authorization for…

On Sept. 29, 2023, the U.S. Food and Drug Administration announced it was taking steps to help further…

On Sept. 28, 2023, Amicus Therapeutics announced that the U.S. Food and Drug Administration (FDA) had approved Pombilitiル…

On Sept. 26, 2023, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) announced…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…
On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…
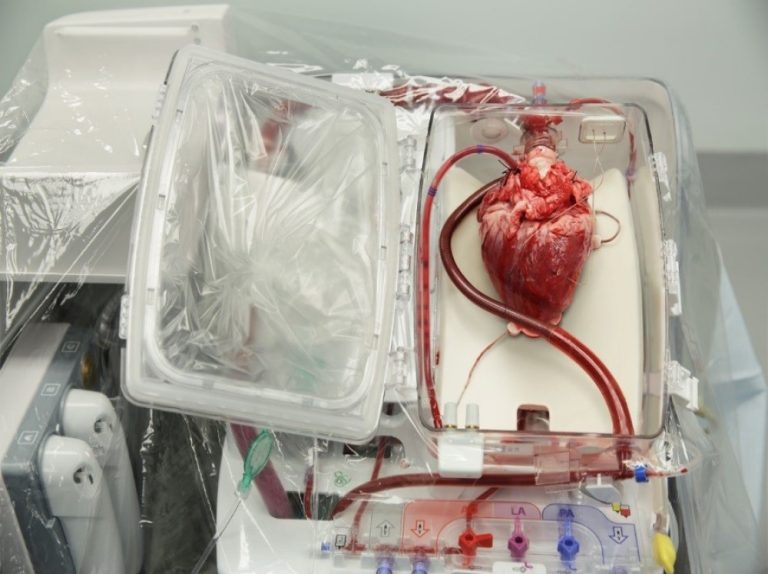
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 4, 2023, Biogen and Sage Therapeutics announced that the U.S. Food and Drug Administration (FDA) had…
On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…
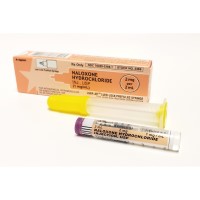
On Jul. 28, 2023, the U.S. FDA approved RiVive, 3 milligram (mg) naloxone hydrochloride nasal spray for over-the-counter…

On Jul. 20, 2023, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved CYFENDUSル…

On Jul. 17, 2023, the FDA approved AstraZeneca’s Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV)…
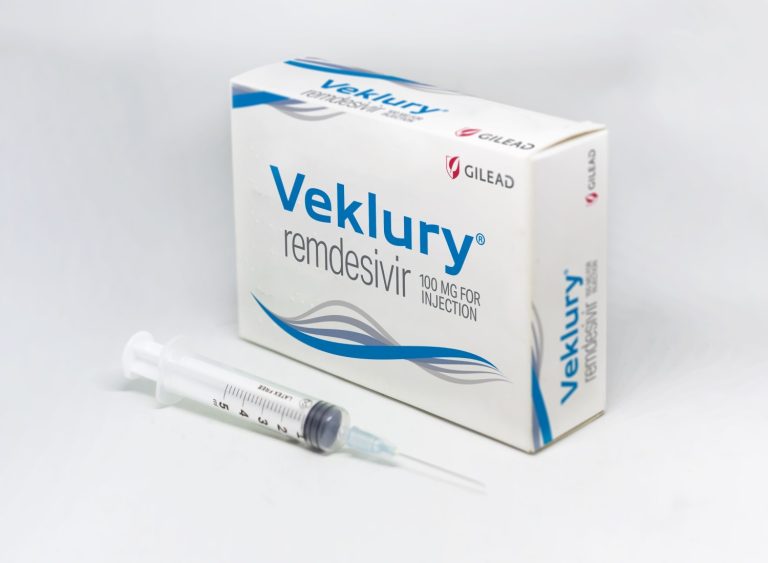
On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 6, 2023, Eisai and Biogen announced that the U.S. Food and Drug Administration (FDA) had approved the…
On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…

On Jun. 22, 2023 AquaBounty Technologies announced that the Company will pause the construction of its farm in…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On Jun. 20, 2023, the U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim’s Jardiance (empagliflozin) and Synjardy…
On Jun. 16, 2023, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
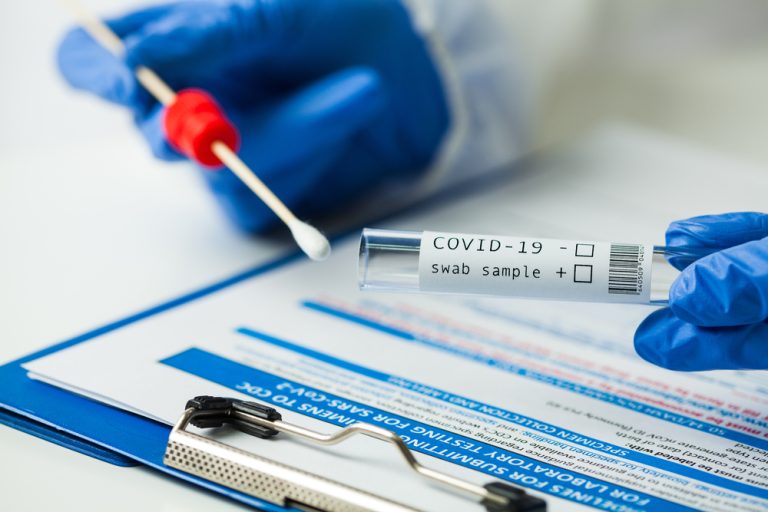
On Jun. 6, 2023, the U.S. Food and Drug Administration (FDA) granted granted marketing authorization for the Cue COVID-19…
On May 31, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…