Pfizer provided U.S. Government with 10 million treatment courses of oral antiviral candidate to help combat COVID-19
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 17, 2021, GlaxoSmithKline and Vir Biotechnology announced U.S. government contracts totalling approximately $1 billion (USD) to…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 11, 2021, Innovation Pharma reported topline results from the Companyメs Phase 2 clinical trial of Brilacidin…

On Nov. 10, 2021, Meridian Bioscience announced that their Revogeneᆴ SARS-CoV-2 assay was granted Emergency Use Authorization by…

On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 9, 2021, Inovio Pharma announced that the U.S. Food and Drug Administration (FDA) provided authorization to…

On Nov. 8, 2021, The U.S. Food and Drug Administration (FDA) reissued the emergency use authorization (EUA) for…

On Nov. 5, 2021, the U.S. Food and Drug Administration The FDA issued an emergency use authorization (EUA)…
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…

On Nov. 1, 2021, the U.S. Food and Drug Administration (FDA) cleared the first 510(k) for a COVID-19…

On Oct. 31, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had notified the Company…
On Oct. 29, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had authorized…
On Oct. 26, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administrationメs (FDA) Vaccines and…

On Oct. 25, 2021, Moderna announced positive interim data from the Phase 2/3 study, called the KidCOVE study,…

On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…

On Oct. 20, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…
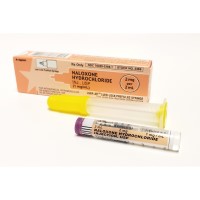
On Oct. 18, 2021, the Food and Drug Administration approved ZIMHI (naloxone hydrochloride) injection as an additional option…

On Oct. 15, 2021, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Tecentriq (atezolizumab)…

On Oct. 14, 2021, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Oct. 14, 2021, Regeneron announced that the U.S. Food and Drug Administration (FDA) had accepted for priority…

On Oct. 11, 2021, Merck and Ridgeback Biotherapeutics announced that Merck had submitted an Emergency Use Authorization (EUA)…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Oct. 5, 2021, Johnson & Johnson announced it had submitted data to the U.S. Food and Drug…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…

On Oct. 1, 2021, Kite, a Gilead Company, announced the U.S. Food and Drug Administration had granted approval…

On Sept. 30 2021, Siemens Healthineers announced the Food and Drug Administration (FDA) 510 (k) clearance of the…