$81 million was awarded by NIAID through four new contracts to support development of candidate HIV vaccines
On Sept. 29, 2003, $81 million was awarded by National Institute of Allergy and Infectious Diseases (NIAID) through…
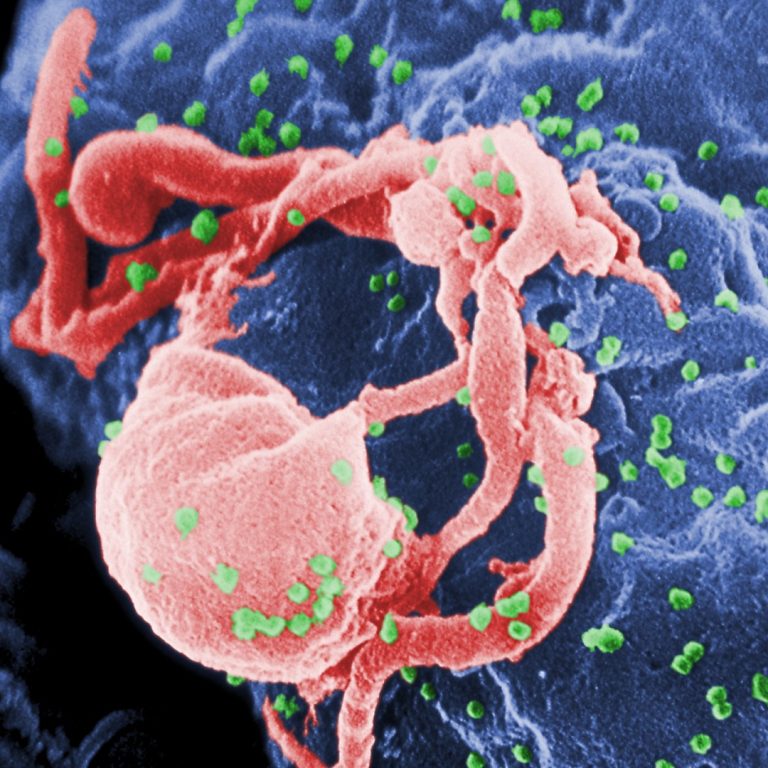
On Sept. 29, 2003, $81 million was awarded by National Institute of Allergy and Infectious Diseases (NIAID) through…
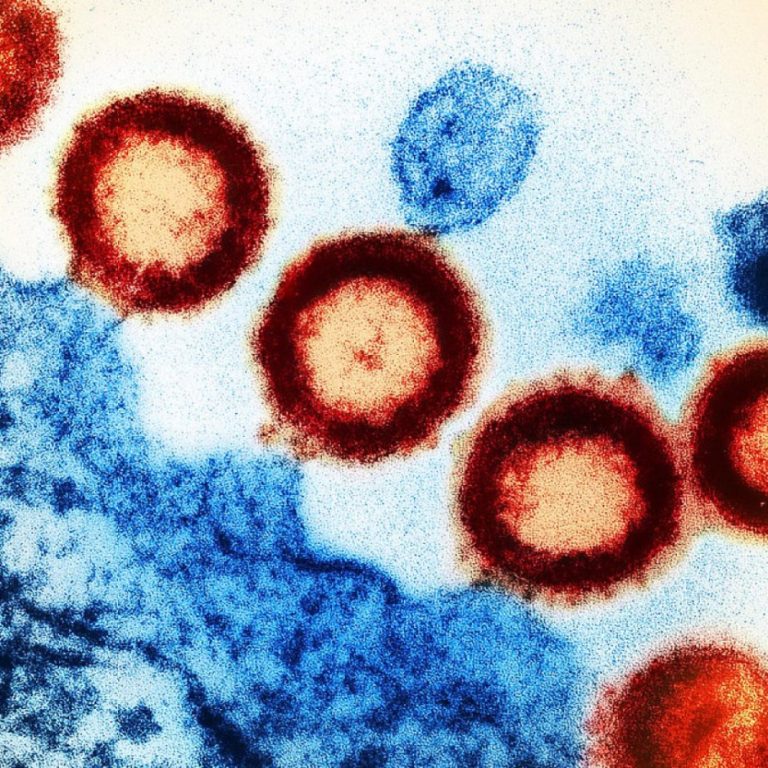
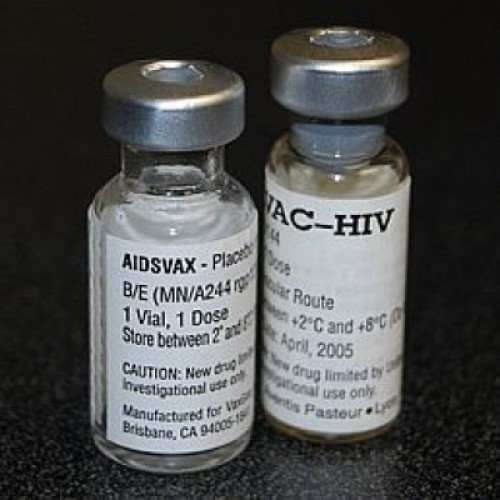
On February 24 2003, VaxGen announced that AIDSVAX B/B did not prove effective in the trials conducted in…

On Jan. 26, 2003, President Bush, in his State of the Union Address, announced the Emergency Plan for…
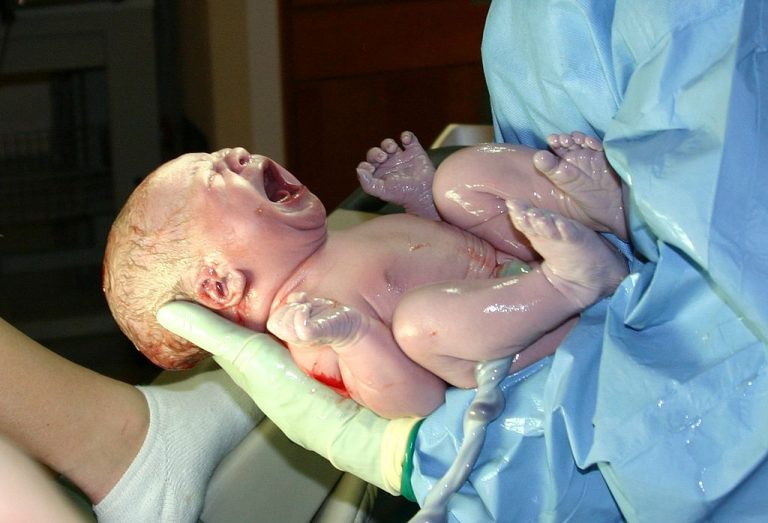
On Jul. 9, 2002, the U.S. Centers for Disease Control and Prevention (CDC) reported newborn HIV infection reductions…

On Feb. 28, 2002, the FDA announced it had licensed the first nucleic acid test (NAT) system intended…

In 2002, Stanford geneticist Mark Kay uses a gene-therapy technique known as RNA inhibition to switch off genes…
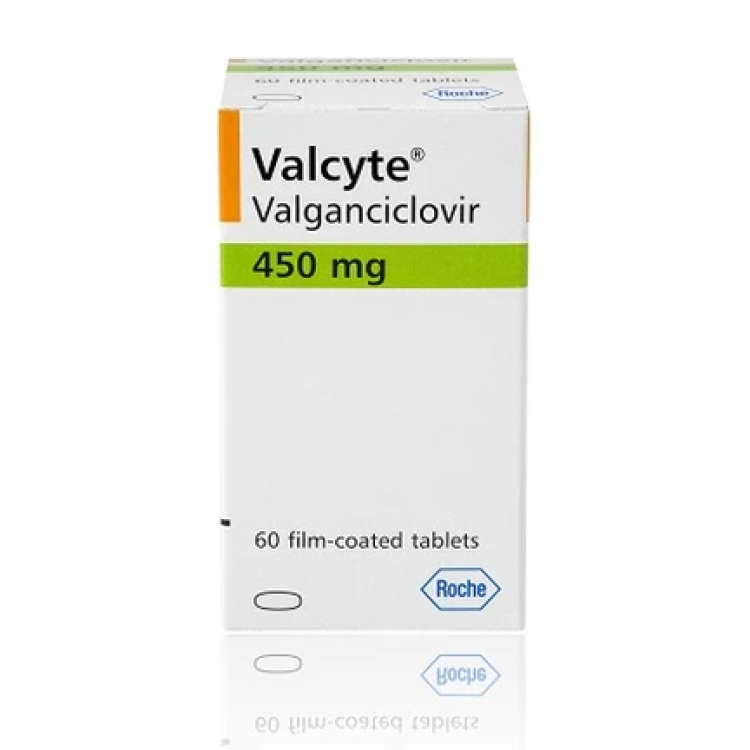
On Mar. 29, 2001, the Food and Drug Administration (FDA) announced it had approved Genentech’s drug Valcyte (valganciclovir…

On May 25, 2000, the National Institute of Allergy and Infectious Diseases (NIAID) announced funding of nine U.S….
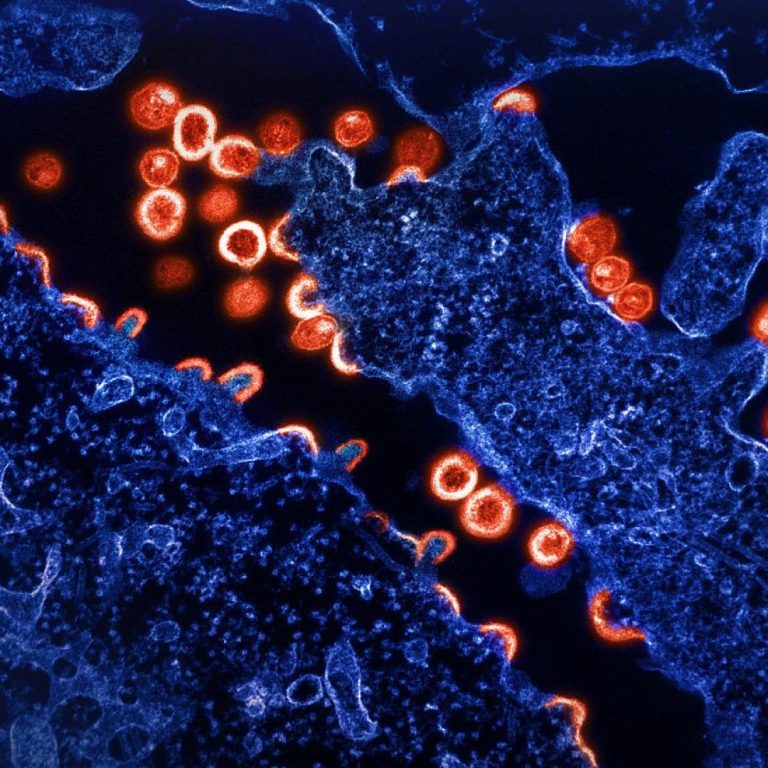
On Apr. 1, 2000, the Centers for Disease Control and Prevention (CDC) announced that it had expanded the…

On Feb. 7, 1999, National Black HIV/AIDS Awareness Day was founded by five national organizations funded by the…

On Jan. 1, 1999, HIV infection in adults (clients 13 years of age or older) became reportable by…

On Jun. 18, 1996, the International AIDS Vaccine Initiative (IAVI) was launched, calling for the speedy development of…
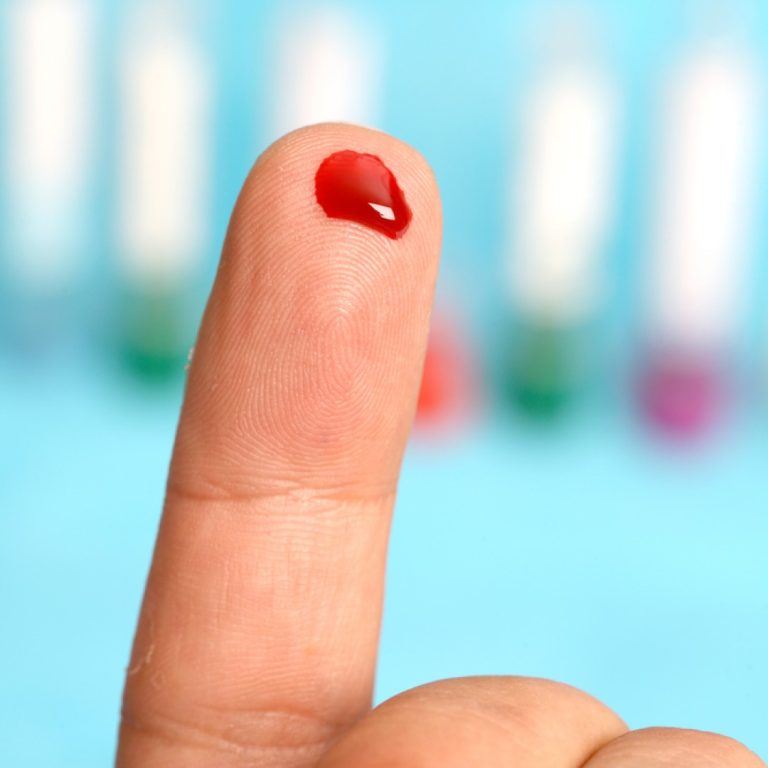
On Mar. 14, 1996, the U.S. Food and Drug Administration (FDA) announced the approval of the first antigen…

In 1995, AIDS researcher David D. Ho from the The Rockefeller University unlocked HIV replication that led to…

In 1995, the Public Health Service (PHS) published guidelines for zidovudine (ZDV) used to reduce perinatal human immunodeficiency…

On Dec. 23, 1994, the FDA announced the approval of the first U.S. HIV test system using oral…
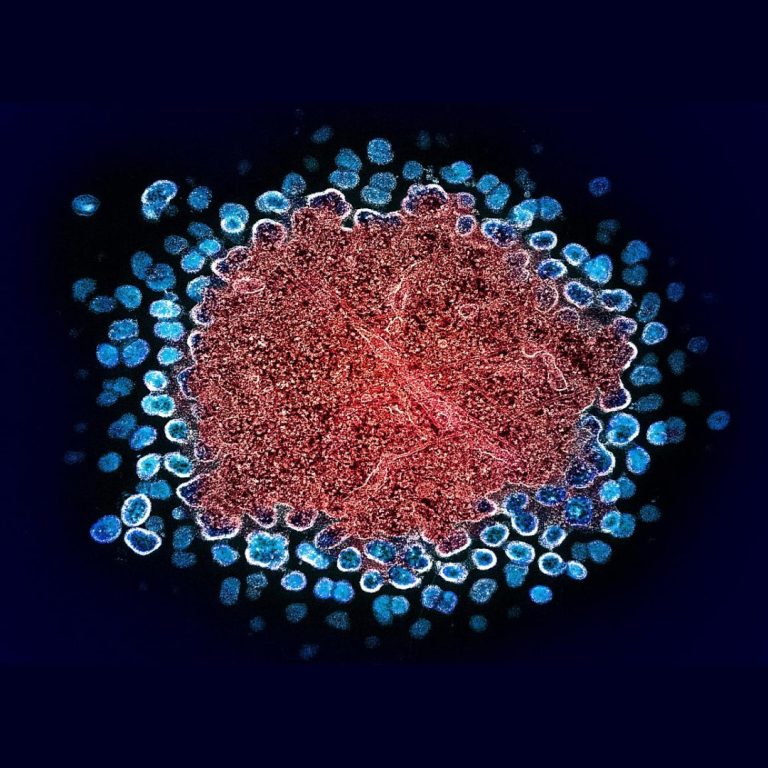
In 1993, The U.S. Centers for Disease Control and Prevention (CDC) revised its definition of AIDS to include…

In 1993, Cook County Hospital’s HIV/AIDS clinic was re-named the Sable/Sherer Clinic. The clinic treated one-third of Cook…

In 1993, Targeted Genetics, a subsidiary of the Immunex Corporation, became the first company to begin human clinical…
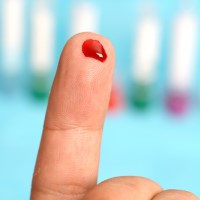
On Jun. 1, 1992, the FDA recommended that all donated blood be screened for antibodies to human immunodeficiency…

In Apr. 13, 1982, Representative Henry Waxman, U.S. Congressman from Los Angeles, held the first congressional hearing on…

On Oct. 9, 1991, Bristol-Myers’ VIDEX (didanosine) was approved by the U.S. Food and Drug Administration for the…
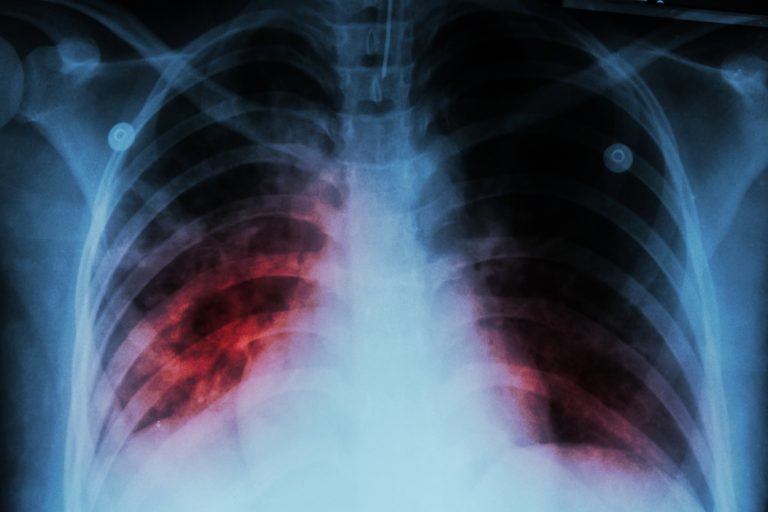
In 1991, a sharp increase of tuberculosis was reported by the U.S. Centers for Disease Control and Prevention…

On Aug. 18, 1990, the Ryan White Comprehensive AIDS Resources Emergency Act was signed into law by President…
In 1989, McGill University researcher Dr. Bernard Belleau developed the antiviral drug 3TC (Lamivudine), which became a critical…

On Dec. 1, 1988, World AIDS Day is held on December 1 each year and is an opportunity…

On Jun. 25, 1988, the U.S. Postal Service (USPS) proposed to ban mailings of microbe samples capable of…

In 1988, the Center for AIDS Research at Albert Einstein Cancer Center was funded by the National Institutes…

In 1987, the U.S. Centers for Disease Control and Prevention (CDC) released the results of a study on…

On Feb. 1, 1987, the World Health Organization (WHO) launched its Global Program on AIDS as the architect…