From lung scarring to heart damage, COVID-19 may leave lingering marks
On Jul. 8, 2020, scientists at the UC Berkeley-UCSF Joint Medical Program reported that for some individuals with…
On Jul. 8, 2020, scientists at the UC Berkeley-UCSF Joint Medical Program reported that for some individuals with…

On Jul. 8, 2020, Moderna announced that it had completed enrollment for both cohorts of the Phase 2…
On Jul. 8, 2020, BD (Becton, Dickinson) announced the formation of a strategic, public-private partnership with the Biomedical…
On Jul. 8, 2020, the European Commission announced regulatory approval for the first adjuvanted quadrivalent influenza vaccine to…

On Jul. 8, 2020, the Fred Hutchinson Cancer Research Center was named the coordinating center for vaccine clinical…

On Jul. 8, 2020, scientists from Texas A&M University, biopharmaceutical company Pulmotect and MD Anderson Cancer Center announced…

On Jul. 7, 2020, Novavax announced that it had been selected to participate in Operation Warp Speed, a…

On Jul. 7, 2020, Regeneron announced that, as part of Operation Warp Speed, the Biomedical Advanced Research and…

On Jul. 7, 2020, Tonix Pharmaceuticals announced its intent to purchase an approximately 40,000 square foot facility in…
On Jul. 6, 2020, Anixa Biosciences announced that it and partner OntoChem had completed the initial in silico…
On Jul. 4, 2020, the World Health Organization (WHO) accepted the recommendation from the Solidarity Trial’s International Steering…

On Jul. 3, 2020, CytoDyn announced it had signed an exclusive Distribution and Supply Agreement with American Regent…
On Jul. 2, 2020, the U.S. Centers for Disease Control and Prevention (CDC) announced a publication in the…

On Jul. 2, 2020, UConn announced a licensing deal with Connecticut Biotech, a startup company headquartered in South…

On Jun. 30, 2020, Altimmune announced dosing of the first patient in the Company’s Phase 1b clinical trial…
On Jun. 29, 2020, National Institutes of Health (NIH) researchers announced they had discovered that Mediterranean populations may…

On Jun. 29, 2020, Bharat Biotech announced it had successfully developed COVAXIN, India’s 1st vaccine candidate for COVID-19,…

On Jun. 29, 2020, the Michael Smith Foundation for Health Research (MSFHR) COVID-19 Research Response Fund announced funding…
On Jun. 25, 2020, the University of Alberta (U of A) announced a study that will analyze thousands…

On Jun. 25, 2020 Moderna announced a collaboration for large-scale, commercial fill-finish manufacturing of Moderna’s mRNA-based COVID-19 vaccine…
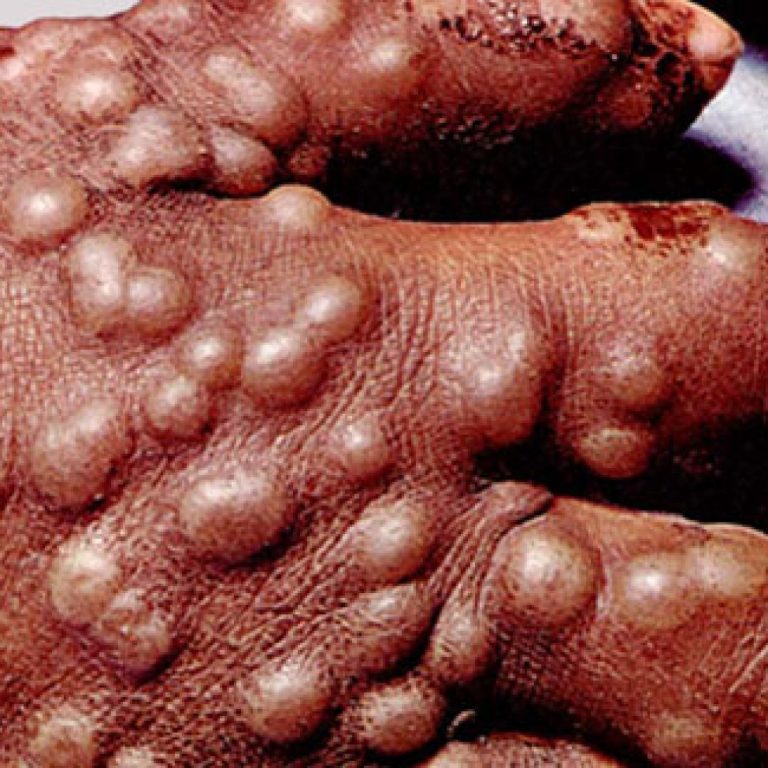
On Jun. 25, 2020, SIGA Technologies announced the deliveries of oral TPOXX (tecovirimat) to the U.S. Department of…

On Jun. 24, 2020, Sinovac Biotech announced the China National Medical Products Administration (NMPA) issued a product license…

On Jun. 24, 2020, Oncotelic, a wholly owned subsidiary of Mateon Therapeutics, announced that IBM has granted access…

On Jun. 24, 2020, a team of scientists at Texas A&M University announced it is working with iBio…

On Jun. 23, 2020, scientists at Scripps Research reported that some common strains of influenza have the potential…

On Jun. 23, 2020, INOVIO announced it had received $71 million funding from the U.S. Department of Defense…

On Jun. 22, 2020, Taconic Biosciences announced an exclusive agreement with the University of Texas Medical Branch at…

On Jun. 20, 2020, the National Institutes of Health (NIH) announced that a clinical trial to evaluate the…

On Jun. 18, 2020, Tonix Pharmaceuticals announced an expansion of its strategic collaboration with Southern Research to include…

On Jun. 17, 2020, BioSig Technologies, and its subsidiary, ViralClear, announced that it had commenced patient enrollment with…