CDC published updated recommendations for prevention of hepatitis A virus infection
On Oct. 19, 2007, the U.S. Centers for Disease Control and Prevention (CDC) published updated recommendations for prevention…
On Oct. 19, 2007, the U.S. Centers for Disease Control and Prevention (CDC) published updated recommendations for prevention…
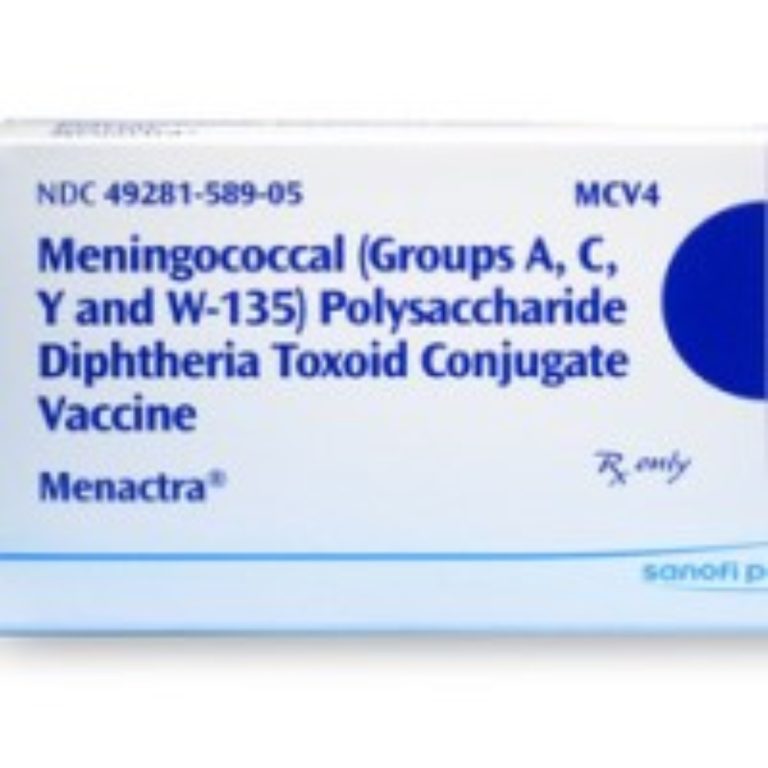
On Oct. 17, 2007, the Food and Drug Administration (FDA) approved quadrivalent meningococcal conjugate vaccine (MCV4) (Menactra®, Sanofi…
On Sept. 28, 2007, the U.S. Food and Drug Administration (FDA) approved an Australian-made influenza vaccine called Afluria…

On Aug 10, 2007, the U.S. Centers for Disease Control and Prevention (CDC) notified MMWR readers of revised…

On Aug. 8, 2007, JAMA reported that research demonstrated a strong association between early, sustained, and layered application…

On Jul. 20, 2007, the U.S. Centers for Disease Control and Prevention (CDC) announced in MMWR that revised…

On Jul. 17, 2007, The U.S. Department of Health and Human Services (HHS) announced it was providing states,…

On Jun. 14, 2007, the U.D. of Health and Human Services (HHS) awarded $132.5 million to Sanofi Pasteur…
On May 8, 2007, the National Cancer Institute (NCI) reported that people infected with the hepatitis C virus…
On Apr. 17, 2007, Sanofi announced that the U.S. Food and Drug Administration (FDA) had licensed its H5N1…
On Mar. 28, 2007, the U.S. Food and Drug Administration (FDA) announced that GlaxoSmithKline (King of Prussia, Pennsylvania)…

In 2007, researchers at Livermore National Laboratory announced they had developed candidate signatures for pathogens that might be…
In January 2007, scientists at the University of Iowa reported in the Journal of Virology that they had…

In 2007, Cocrystal Discovery, now known as Cocrystal Pharma, was founded in Bothell as a pharmaceutical company with…
On Dec. 14, 2006, the U.S. Centers for Disease Control and Prevention (CDC) reported a multi-state outbreak of…

On Feb. 6, 2006, Sanofi pasteur announced it had delivered more H5N1 vaccine to the U.S. government including…

On Feb. 3, 2006, Rotavirus vaccine, live, oral, pentavalent (RotaTeq by Merck) was licensed for use in infants…
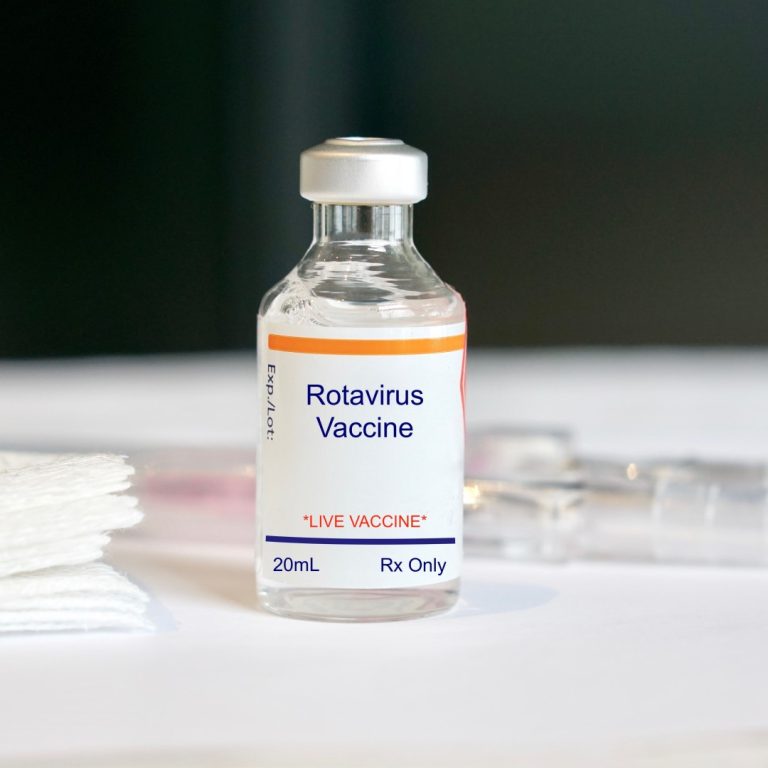
On Jan. 6, 2006, the U.S. Centers for Disease Control and Prevention’s (CDC) advisory Committee on Immunization Practices…
Aug. 24, 2024, the U.S. Centers for Disease Control and Prevention (CDC) activated its Emergency Operations Center (EOC)…

In 2006, the Kansas State University Biosecurity Research Institute dedicated the Biosecurity Research Institute (BRI) that was designed…
In 2006, the Malaria Atlas Project (MAP) was founded as an international research collaboration that tracks the global…
On Dec. 23, 2005, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
On Dec. 19, 2005, in a 2-year legal battle over the U.S. military’s anthrax vaccination program, the Food…

On Oct. 30, 2005, the Bill & Melinda Gates Foundation announced three grants totaling $258.3 million for advanced…

On Sept. 6, 2005, the vaccine that combined the measles, mumps, rubella, and varicella antigens (Proquad by Merck)…
On Aug. 31, 2005, the inactivated, injectable influenza vaccine (Fluarix) by GlaxoSmithKline (GSK) was licensed. The vaccine was…
On Aug. 11, 2005, the Food and Drug Administration (FDA) approved an application of a pediatric/adolescent formulation of…
On Jun. 9, 2005, the U.S. Food and Drug Administration (FDA) licensed a 2nd Tdap vaccine (Adacel by…

On Apr. 22, 2005, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had granted licensure to…
On Apr. 1, 2005, Sanofi pasteur was awarded a five-year $97 million contract from the U.S. Department of…