Sanofi and GSK agreed with the UK government to supply up to 60 million doses of COVID-19 vaccine
On Jul. 29, 2020, Sanofi and GSK announced an agreement with the UK government for the supply of…
On Jul. 29, 2020, Sanofi and GSK announced an agreement with the UK government for the supply of…
On Jul. 27, 2020, MediciNova announced an agreement with BioComo and Mie University (Mie prefecture, Japan) for joint…

On Jul. 16, 2020, Dynavax Technologies and the Icahn School of Medicine at Mount Sinai announced they had…

On Jul. 15, 2020, Tevogen Bio announced its intention to evaluate its proprietary antigen-specific T cell technology as…

On Jul. 13, 2020, Tonix Pharmaceuticals announced a new preclinical research and option agreement with Kansas State University…
On Jul. 8, 2020, the European Commission announced regulatory approval for the first adjuvanted quadrivalent influenza vaccine to…

On Jul. 6, 2020, the FDA issued an emergency use authorization (EUA) for the third diagnostic test for…

On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
On Jul. 2, 2020, the U.S. Centers for Disease Control and Prevention (CDC) announced a publication in the…

On Jun. 24, 2020, Sinovac Biotech announced the China National Medical Products Administration (NMPA) issued a product license…

On Jun. 23, 2020, scientists at Scripps Research reported that some common strains of influenza have the potential…

On Jun. 11, 2020, Quidel announced it had received funding from the Biomedical Advanced Research and Development Authority…
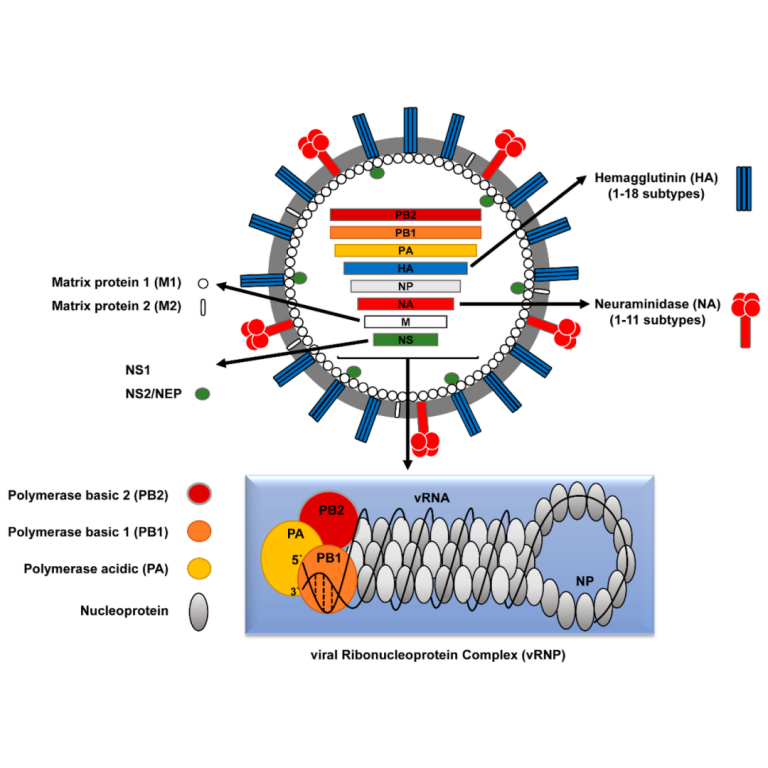
On Jun. 8, 2020, Intravacc and Versatope announced that they have signed a research service agreement to further…

On May 6, 2020, Baxter announced results of the Fluid Response Evaluation in Sepsis Hypotension and Shock (FRESH)…

On May 6, 2020, Predictive Oncology announced the acquisition of Soluble Therapeutics and partnership and licensing of a…
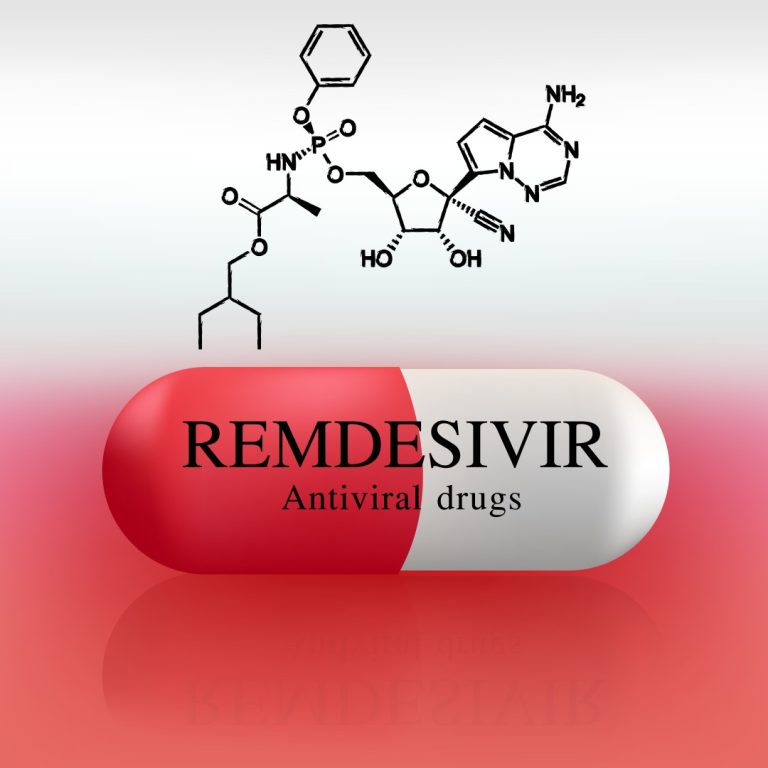
On Apr. 29, 2020, Emory University played a leading role in the government-sponsored clinical trial of the COVID-19…
On Apr. 27, 2020, BioAegis Therapeutics announced it had published gene expression data in animal studies where recombinant…

On Apr. 17, 2020, Celdara Medical announced that it had entered into a Cooperative Research and Development Agreement…

On Apr. 15, 2020, FUJIFILM announced that it had expanded its manufacturing capacity at FUJIFILM Toyama Chemical Co.,…

On Apr. 9, 2020, FUJIFILM announced initiation of a U.S. phase II clinical trial to evaluate the safety…

On Apr. 9, 2020, the U.S. Department of Agriculture (USDA) confirmed the presence of highly pathogenic H7N3 avian…
On Apr. 8, 2020, Nanomix announced that the company had been awarded $570,000 in funding from Biomedical Advanced…

On Apr. 7, 2020, the U.S. Food and Drug Administration (FDA) approved an Investigational New Drug application by…
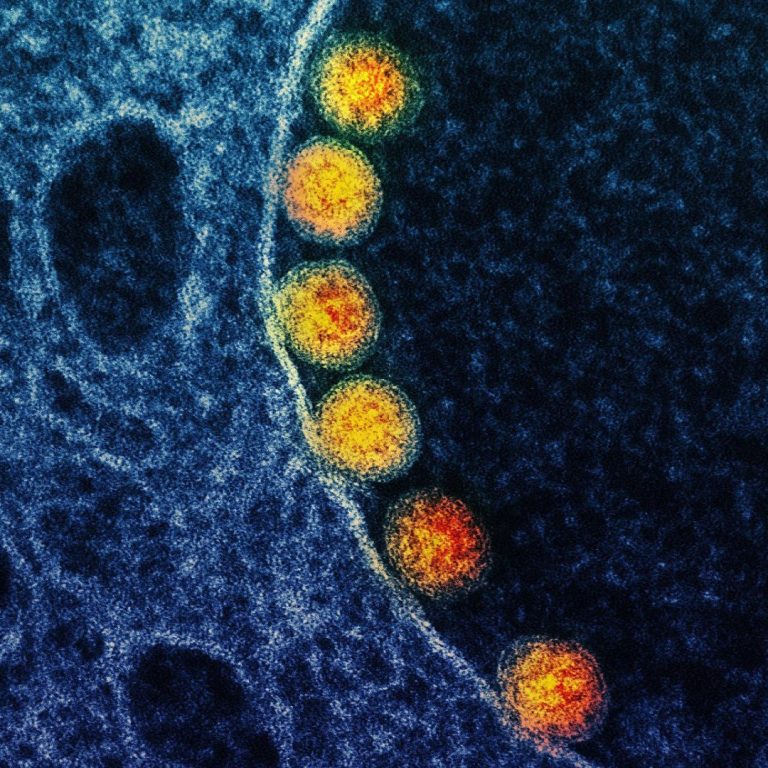
On Apr. 4, 2020, Mesa Biotech announced the addition of the novel coronavirus (SARS-CoV-2) to its active influenza…

On Apr. 2, 2020, an international collaboration of virologists at the University of Wisconsin-Madison and the vaccine companies…

On Mar. 31, 2020, FUJIFILM announced initiation of a phase III clinical trial to evaluate the safety and…

On Mar. 31, 2020, Cue Health announced that is had been awarded up to $30 million in base…

On Mar. 31, 2020, Emergent BioSolutions announced an agreement with Novavax whereby Emergent provided molecule-to-market contract development and…
On Mar. 26, 2020, Amyris announced that it had completed initial testing of a leading vaccine adjuvant. In…
On Mar. 24, 2020, Novavax announced positive top-line results of its pivotal Phase 3 clinical trial of NanoFlu,…