Salk Institute to lead $126 million effort to map the aging human brain
On Supt. 22, 2022, with a five-year, $126 million grant from the National Institutes of Health (NIH), a…

On Supt. 22, 2022, with a five-year, $126 million grant from the National Institutes of Health (NIH), a…

On Sept. 21, 2022, the AACR released the 12th edition of its AACR Cancer Progress Report, an annual…

On Sept. 20, 2022, the University of California, Davis announced that the amphibian decline in Latin America in…
On Sept. 15, 2022, Gilead Sciences announced updates to the World Health Organizationメs Therapeutics and COVID-19: living guideline,…

On Sept. 15, 2022, the National Institutes of Health announced that a research team funded by the NIH…
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…

On Sept. 13, 2022, the National Institutes of Health announced it will invest $130 million over four years,…

On Sept. 12, 2022, the White House announced an executive order to advance biotechnology and biomanufacturing towards innovative…

On Sept. 8, 2022, the U.S. Department of Agricultureメs Animal and Plant Health Inspection Service launched an updated…

On Sept. 8, 2022, researchers from the University of Oxford and their partners reported findings from their Phase…

On Sept. 7, 2022, Quest Diagnostics announced that it had received emergency use authorization (EUA) from the U.S….
On Sept. 6, 2022, scientists in China announced that they had conducted the first comprehensive study of the…

On Aug. 29, 2022, a group of international researchers, coordinated by Dr Jo�o Teixeira at The Australian National…

On Aug. 23, 2022, Pfizer and BioNTech announced updated efficacy results from a Phase 2/3 trial evaluating a…

On Aug. 23, 2022, Health authorities in the Democratic Republic of the Congo declared a resurgence of Ebola…
On Aug. 22, 2022, Pfizer and BioNTech announced they had completed a submission to the U.S. Food and…
On Aug. 18, 2022, the White House announced that the HHS had accelerated Phase 4 of its National…

On Aug. 17, 2022, the U.S. Food and Drug Administration approved Zynteglo (betibeglogene autotemcel) developed by bluebird bio,…

On Aug. 16, 2022, UNICEF announced that it had awarded a contract for the first ever supply of…

On Aug. 15, 2022, Roche announced the launch of the Elecsysᆴ IGRA SARS-CoV-2 test in countries that accept…
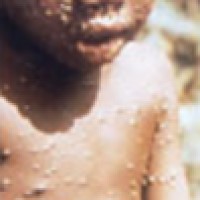
On Aug. 15, 2022, the U.S. Department of Health and Human Services announced that was making up to…
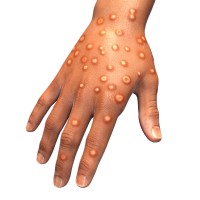
On Aug. 12, 2022, a group of global experts convened by WHO agreed on new names for monkeypox…
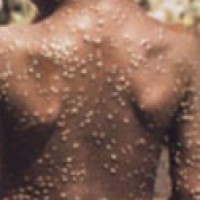
On Aug. 9, 2022, the U.S. Food and Drug Administration issued an emergency use authorization for the JYNNEOS…

On Aug. 9, 2022, the the Department of Health and Human Services (HHS) announced it had determined that…

On Aug. 5, 2022, researchers from the National Institutes of Health announced that they had developed a three-dimensional…
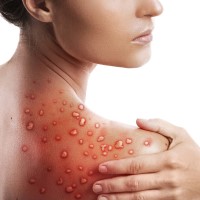
On Aug. 4, 2022, the U.S. Department of Health and Human Services declared the ongoing spread of monkeypox…

On Jul. 30, 2022, scientists announced they had sequenced the genome of a 6,000-year-old Citrullus seeds that revealed…

On Jul. 27, 2022, BD (Becton, Dickinson) announced their newly developed molecular polymerase chain reaction (PCR) test for…

On Jul. 27, 2022, Pfizer and BioNTech announced that the companies had initiated a randomized, active-controlled, observer-blind, Phase…
On Jul. 22, 2022, Gilead Sciences announced that the Committee for Medicinal Products for Human Use (CHMP) of…