Roche developed unique PCR tests to detect the monkeypox virus
On May 25, 2022, Roche and its subsidiary TIB Molbiol announced they had developed three unique LightMixᆴ Modular…
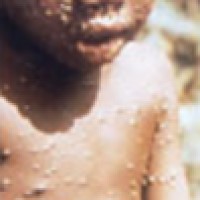
On May 25, 2022, Roche and its subsidiary TIB Molbiol announced they had developed three unique LightMixᆴ Modular…

On May 25, 2022, the Health and Human Servicesagency (HHS) announced the formal establishment of the Advanced Research…

On May 24, 2022, scientists at the Broad Institute of MIT and Harvard and the University of Massachusetts…

On May 23, 2022, Jaguar Health announced the launch of Canine Cancer: Take C.H.A.R.G.E. (Canine Health And ReGistry…

On May 21, 2022, the WHO announced that since May 13, 2022, cases of monkeypox had been reported…

On May 19, 2022, WHO issued an emergency use listing (EUL) for CONVIDECIA, a vaccine manufactured by CanSino…

On May 18, 2022, the U.S. National Institutes of Health announced it had awarded approximately $577 million to…
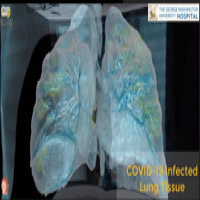
On May 18, 2022, SARS-CoV-2, or at least pieces of it, sticks around longer in some infected individuals…
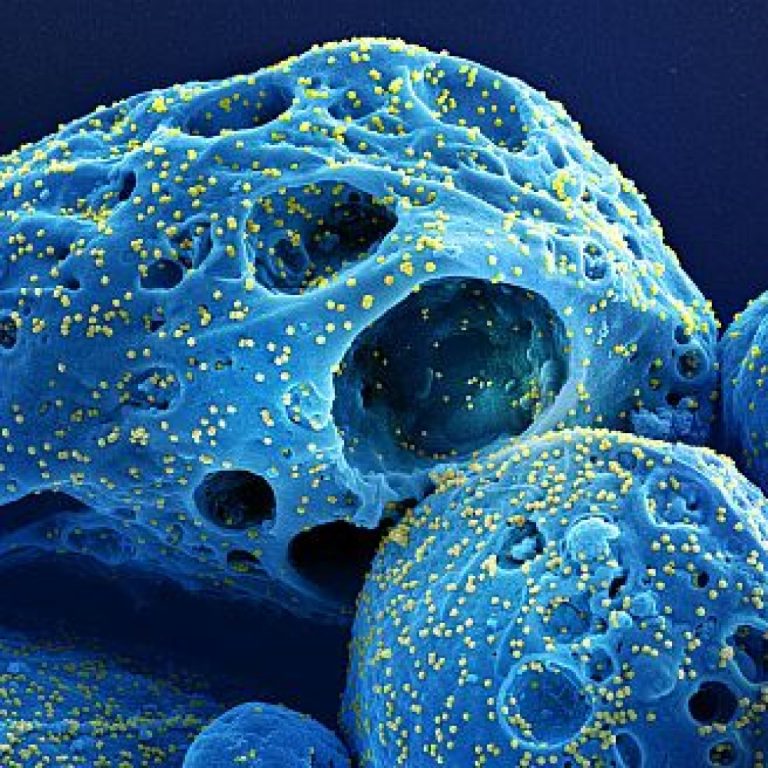
On May 18, 2022, researchers at Gladstone Institutes and UC San Francisco (UCSF) reported that unvaccinated people, infection…
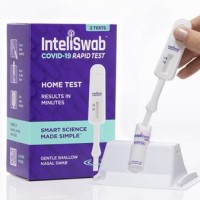
On May 17, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests detect the Omicron BA.2, BA.2.12.1,…
On May 17, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration expanded emergency use authorization…

On May 16, 2022, working with state and federal wildlife agencies and private and public zoos across Arizona,…

On May 12, 2022, WHOメs COVID-19 Technology Access Pool and the Medicines Patent Pool finalized a licensing agreement…

On May 12, 2022, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On May 12, 2022, the U.S. National Institutes of Health announced that it had licensed 11 COVID-19 research…

On May 11, 2022, Lucira Health announced that it had submitted a request to the U.S. Food and…

On May 9, 2022, Lucira Health announced that both its COVID-19 & Flu and COVID-19 molecular tests have…
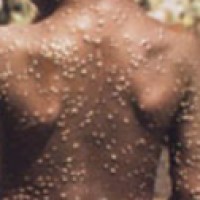
On May 7, 2022, WHO was informed of a confirmed case of monkeypox in an individual who travelled…

On May 6, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) announced that it had launched…

On May 5, 2022, the World Health Organization (WHO) reported estimates that show the full death toll associated…

On May 4, 2022, Oxitec announced approval from the Florida Department of Agriculture and Consumer Services, including reviews…

On Apr. 27, 2022, the U.S. Department of Agricultureメs Animal and Plant Health Inspection Service announced it had…

he Evolution of DNA is an Illustration with the very small, requiring magnification, to the very large. Cast…

On Apr. 25, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Apr. 21, 2022, CureVac announced preclinical data demonstrating immune responses and protective efficacy of a bivalent second-generation…

On Apr. 20, 2022, Codiak BioSciences announced new preclinical data from its pan beta-coronavirus vaccine program, which aims…
On Mar. 14, 2022, Pfizer and BioNTech announced positive results from a Phase 2/3 clinical trial evaluating the…

On Nov. 12, 2021, Regeneron announced that the U.S. Food and Drug Administration had extended by three months…

On Apr. 14, 2022, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the…

On Apr. 14, 2022, Merck announced that V116, the companyメs investigational 21-valent pneumococcal conjugate vaccine, had received Breakthrough…