Leading Generic Drug Makers Unite to Pledge Capacity for Developing and Delivering Affordable COVID-19 Interventions as Pandemic Intensifies
On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…

On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…

On Nov. 12, 2021, Lincoln Children’s Zoo announced on Facebook that their three snow leopards: Ranney, Everest and…

On Nov. 11, 2021, Zipline, Pfizer and BioNTech announced that Zipline had successfully completed the first long-range drone…

On Nov. 11, 2021, Roche announced that the European Medicines Agencyメs (EMA) Committee for Medicinal Products for Human…

On Nov. 11, 2021, Innovation Pharma reported topline results from the Companyメs Phase 2 clinical trial of Brilacidin…

On Nov. 11, 2021, the University of Oxford began recruiting for a Phase I trial to test an…

On Nov. 10, 2021, a study by researchers at Georgia State University showed that the politicization of science…

On Nov. 10, 2021, Meridian Bioscience announced that their Revogeneᆴ SARS-CoV-2 assay was granted Emergency Use Authorization by…

On Nov. 10, 2021, the Biden-Harris Administration announced that it was increasing access to COVID-19 testing for Americans…

On Nov. 9, 2021, Swedish announced a $20 million personal bequest from the late philanthropist Paul G. Allen,…

On Nov. 9, 2021, Novan announced promising preclinical safety results with berdazimer sodium in a 14-day GLP repeat…

On Nov. 9, 2021, Howard Hughes Medical Institute announced the program, named Codetta, can read the genome sequence…

On Nov. 8, 2021, Amyris announced announced it had entered into a 50:50 joint venture arrangement with ImmunityBio…

On Nov. 8, 2021, The U.S. Food and Drug Administration (FDA) reissued the emergency use authorization (EUA) for…

On Nov. 5, 2021, scientists at the National Institutes of Health announced they had found that a process…

On Nov. 5, 2021, scientists at Oxford University announced they had identified the gene responsible for doubling the…

On Nov. 5, 2021, the U.S. Food and Drug Administration The FDA issued an emergency use authorization (EUA)…

On Nov. 5, 2021, Resverlogix announced that it had entered into a cooperation agreement with the Supreme Council…

On Nov. 3, 2021, Innovation Pharma announced that the Company had received confirmation that hard lock of the…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 3, 2021, the World Health Organization (WHO) issued an emergency use listing (EUL) for COVAXINᆴ (developed…

On Nov. 5, 2021, NRx Pharmaceuticals reported a safety update on ZYESAMIᆴ (aviptadil), which was being tested in…
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…
On Nov. 2, 2021, the National Institutes of Health announced support for a four-year follow-up study on the…

On Nov. 1, 2021, for the first time in the U.S., the transmission of COVID-19 from pet parent…

On Nov. 1, 2021, the U.S. Food and Drug Administration (FDA) cleared the first 510(k) for a COVID-19…

On Oct. 30, 2021, Penn Medicine officially opened the doors of its 1.5 million-square-foot future-ready Pavilion as clinical…
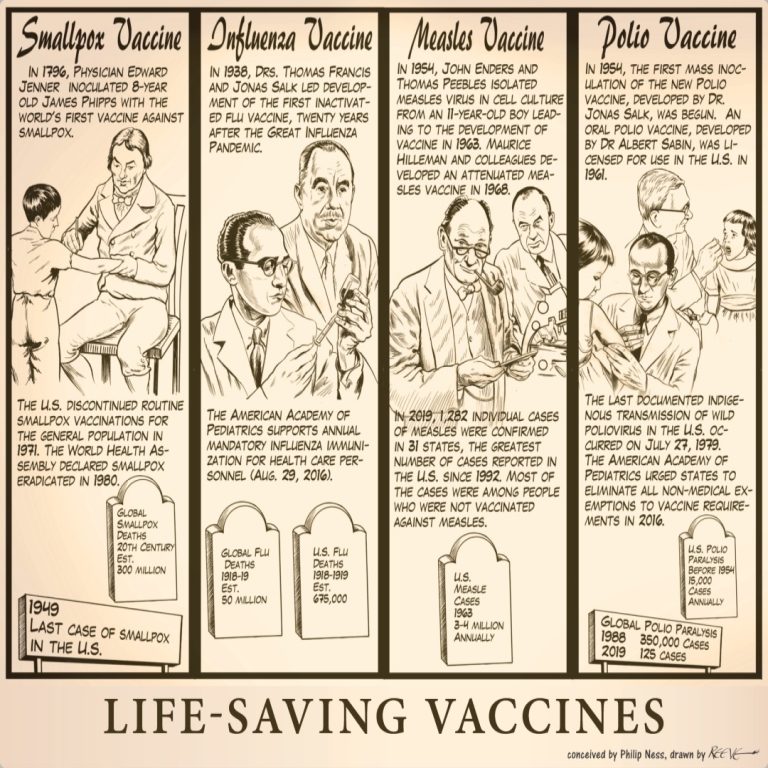
Our Life-Saving Vaccines cartoon illustrates four invaluable vaccines that have affected human civilization throughout history from smallpox and…
On Oct. 29, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had authorized…
On Oct. 29, 2021, research led by scientists at the National Institutes of Health announced they had identified…