UK authorized Regeneron antibody cocktail to prevent and treat acute COVID-19 infection
On Aug. 20, 2021, Regeneron announced that the United Kingdom’s (UK) Medicines and Healthcare products Regulatory Agency had…

On Aug. 20, 2021, Regeneron announced that the United Kingdom’s (UK) Medicines and Healthcare products Regulatory Agency had…
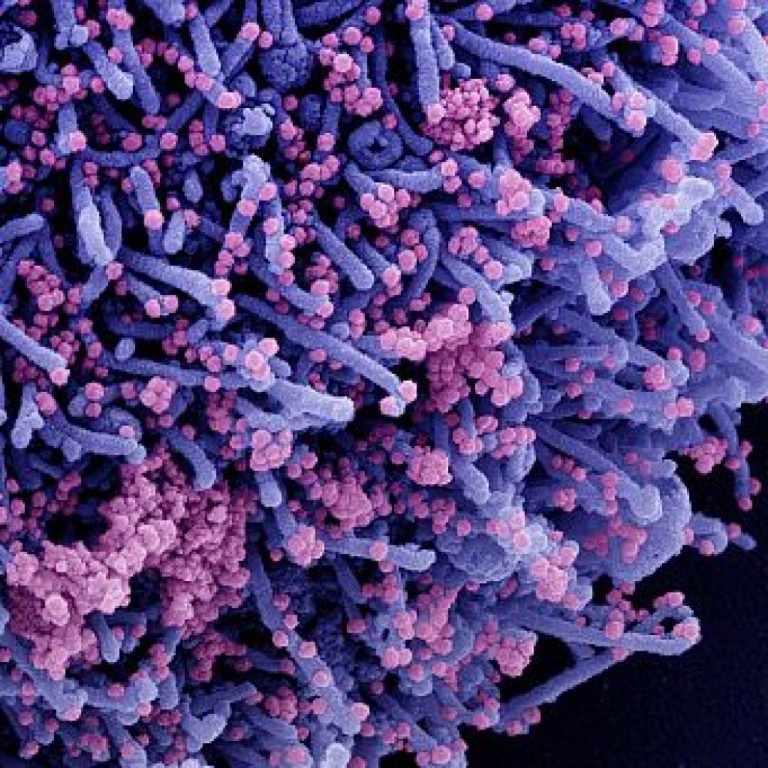
On Aug. 19, 2021, Oregon Health & Science University (OHSU) announced that it was part of a randomized,…

On Aug. 18, 2021, Agilent Technologies announced that researchers at the Indian Institute of Technology Bombay in India…

On Aug. 18, 2021, the final results of the Clinical Trial of COVID-19 Convalescent Plasma in Outpatients (C3PO)…

On Aug. 18, 2021, RELIEF THERAPEUTICS reported that the parent company of its U.S. collaboration partner, NRx Pharmaceuticals,…

On Aug. 17, 2021, Our Future Health published a new tender for the processing, genotyping, and storage of…

On Aug. 16, 2021, ADMA Biologics announced that it had received U.S. Food and Drug Administration approval for…

On Aug. 16, 2021, CureVac and GSK announced publication on the preprint server bioRxiv of preclinical data investigating…

On Aug. 16, 2021, scientists at the National Institutes of Health (NIH) announced they had developed a new…

On Aug. 16, 2021, Pfizer and BioNTech announced that they had submitted Phase 1 data to the U.S….

On Aug. 14, 2021, the Ministry of Health of Cote d’Ivoire confirmed the country’s first case of Ebola…

On Aug. 13, 2021, Rigel Pharma announced that the U.S. Food and Drug Administration (FDA) had informed the…

On Aug. 13, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved TICOVACル (tick-borne…
On Aug. 11, 2021, the National Institutes of Health (NIH) announced that one dose of a new monoclonal…
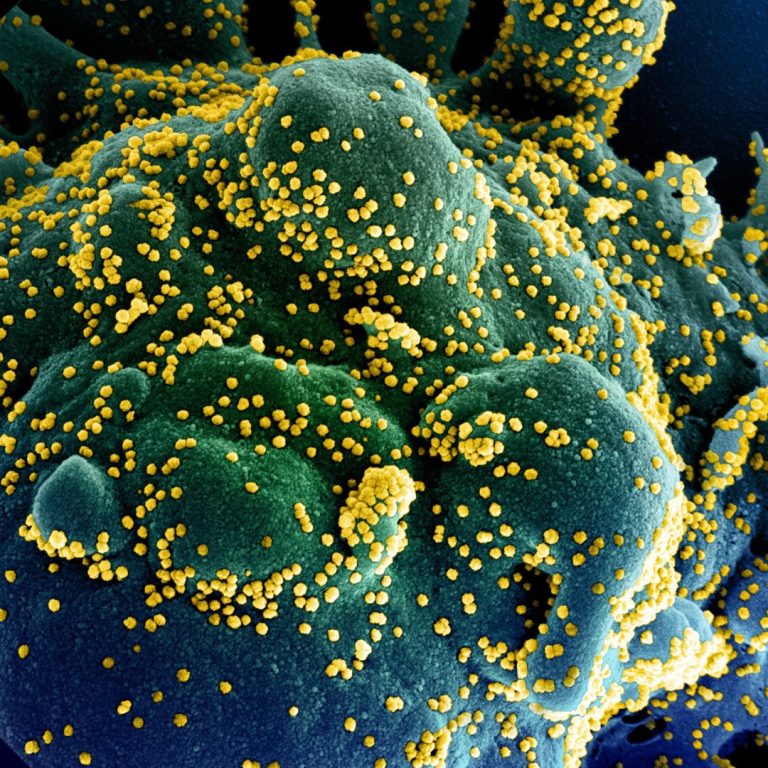
On Aug. 10, 2021, the National Institutes of Health (NIH) announced that a pilot study had begun to…

On Aug. 10, 2021, Cardinal Health announced its efforts to commercialize and broaden access to over-the-counter rapid COVID-19…

On Aug. 6, 2021, the U.S. Food and Drug Administration approved Genzyme’s Nexviazyme (avalglucosidase alfa-ngpt) for intravenous infusion…
On Aug. 6, 2021, the Centers for Disease Control and Prevention (CDC) annouced in MMWR that a study…
On Aug. 5, 2021, an international team of researchers announced that a mutation in a single gene appeared…

On Aug. 4, 2021, Humanigen announced analysis of results from its Phase 3 LIVE-AIR study of lenzilumab in…

On Aug. 4, 2021, Bio-Techne announced that scientists at the U.S. Centers for Disease Control and Prevention (CDC)…

On Aug. 3, 2021, Vanda Pharmaceuticals announced that enrollment had closed in the ODYSSEY study comparing tradipitant and…

On Aug. 3, 2021, Eli Lilly and Incyte announced results from an additional cohort of 101 adult patients…

On Aug. 2, 2021, Vaxart announced that the U.S. Food and Drug Administration had cleared Vaxart’s Investigational New…

On Jul. 30, 2021, the Medicines Patent Pool and the World Health Organization, Afrigen Biologics, the Biologicals and…

On Jul. 30, 2021, Humanigen announced that the NIH had advanced the ACTIV-5/BET-B study to a Phase 2/3…

On Jul. 30, 2021, Regeneron announced that the FDA had updated the Emergency Use Authorization (EUA) for the…

On Jul. 29, 2021, the National Science Foundation announced the establishment of 11 new NSF National Artificial Intelligence…

On Jul. 29, 2021, the University of Oxfordメs and AstraZeneca announced that one billion doses of the ChAdOx1…

On Jul. 28, 2021, the National Institutes of Health announced that by studying lab-grown neurons derived from skin…