NIH announced restructured HIV clinical trials networks
On Nov. 30, 2020, The National Institute of Allergy and Infectious Diseases (NIAID) announced the clinical investigators and…

On Nov. 30, 2020, The National Institute of Allergy and Infectious Diseases (NIAID) announced the clinical investigators and…

On Nov. 27, 2020, SmartPharm Therapeutics, a wholly-owned subsidiary of Sorrento Therapeutics, announced that it had been awarded…

On Nov. 25, 2020, the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes…

On Nov. 25, 2020, AIM ImmunoTech announced that Roswell Park Comprehensive Cancer Centerメs Phase 1/2a study evaluating the…

On Nov. 25, 2020, Altimmune announced that it had submitted an Investigational New Drug (IND) application to the…

On Nov. 25, 2020, BioNTech and Shanghai Fosun Pharmaceutica jointly announced that their lead mRNA COVID-19 vaccine candidate…

On Nov. 25, 2020, a University of Saskatchewan research team announced it had sequenced the genomes for 15…

On Nov. 24, 2020, National Institutes of Health researchers reported they had found that the brainメs protective membranes…
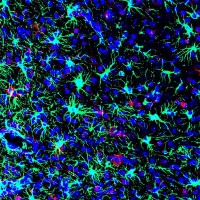
On Nov. 24, 2020, preclinical study by the National Institutes of Health (NIH) suggested FDA-approved tetracycline-based antibiotics may…
On Nov. 24, 2020, JAMA reported that fewer than 10% of people in tye U.S. as of September…

On Nov. 24, 2020, MediciNova announced development progress on its intranasal SARS-CoV-2 vaccine for COVID-19 utilizing BC-PIV, a…

On Nov. 24, 2020, Baxter announced a $50 million expansion of its sterile fill/finish manufacturing facilities located in…

On Nov. 23, 2020, the University of Oxford, in collaboration with AstraZeneca, announced interim trial data from its…

On Nov. 23, 2020, AstraZeneca announced positive high-level results from an interim analysis of clinical trials of AZD1222…

On Nov. 23, 2020, Merck, known as MSD outside the U.S. and Canada, announced the company had submitted…

On Nov. 23, 2020, Viatris announced tentative approval from the FDA for a New Drug Application for pediatric…

On Nov. 23, 2020, Bellerophon Therapeutics announced that the independent Data Monitoring Committee (DMC) had completed its pre-specified…

On Nov. 23, 2020, National Resilience, founded by ARCH Venture Partnersメ Robert Nelsen, launched as the worldメs most…

On Nov. 21, 2020, the FDA issued an emergency use authorization (EUA) for Regeneron Pharmaceuticals’ casirivimab and imdevimab…

On Nov. 21, 2020, Regeneron announced that the antibody cocktail casirivimab and imdevimab administered together (also known as…

On Nov. 20, 2020, the National Institutes of Health (NIH) awarded nearly $45 million to expand the research…

On Nov. 20, 2020, the University of Oxford announced a study suggested that individuals who have previously had…
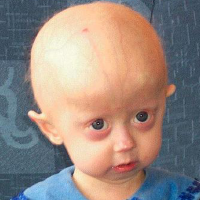
On Nov. 20, 2020, Eiger BioPharmaceuticals announced that the FDA had approved ZokinvyTM (lonafarnib) for the treatment of…

On Nov. 20, 2020, Eli Lilly announced that Health Canada had granted authorization under the Interim Order Respecting…

On Nov. 30, 2020, Hologic announced that the FDA had approved a diagnostic claim for its HIV-1 (human…

On Nov. 20, 2020, researchers at the Broad Institute of MIT and Harvard announced they had developed a…

On Nov. 20, 2020, Pfizer and BioNTech announced they submitted a request to the Food and Drug Administration…

On Nov. 19, 2020, F4 Pharma announced the inclusion of the first patient with severe COVID-19 in a…

On Nov. 19, 2020, the University of Oxford announced that the ChAdOx1 nCov-2019 coronavirus vaccine had been shown…

On Nov. 19, 2020, ApiJect Systems, a public benefit corporatio, announced that it had been approved by the…