National Colorectal Cancer Awareness Month was founded
On Feb. 29. 2000, President Clinton officially dedicated March as National Colorectal Cancer Awareness Month. Since then, it…

On Feb. 29. 2000, President Clinton officially dedicated March as National Colorectal Cancer Awareness Month. Since then, it…
On Feb. 17, 2000, the Food and Drug Administration (FDA) announced it it had licensed a 13-valent pneumococcal…

On Jan. 17, 2000, Glaxo Wellcome announced merger with SmithKline Beecham forming GlaxoSmithKline. Glaxo SmithKline will be the…

In 2000, QB3 founded as the University of California’s hub for innovation and entrepreneurship in life science, with…
On Nov. 15, 1999, the FDA approved LASIK corrective eye surgery. LASIK stands for Laser-Assisted In Situ Keratomileusis…

On Oct. 22, 1999, the Advisory Committee on Immunization Practices (ACIP), after a review of scientific data from…

On Nov. 23, 199, the National Cancer Institute awarded nearly $8 million in grants toward the creation of…

On Aug. 29, 1999, Merck, West Point, Pennsylvania) received approval from the FDA of a supplement to Merck’s…
In August 1999, an outbreak of encephalitis caused by West Nile virus (WNV) was detected in New York…

On Jul. 11, 1999, Daiichi Sankyo and Eli Lilly announced the FDA approved Effientル(prasugrel) tablets for the reduction…

In Jul. 1999, the Biotechnology Industry Organization held BIO’99 in Seattle and the event attracted more than 5,700…

On Jun. 9, 1999, the Dale and Betty Bumpers Vaccine Research Center (VRC) was established at the National…
On Jun. 7, 1999, NeoPath merged with AutoCyte to form TriPath Imaging based in Burlington, N.C. NeoPath was…
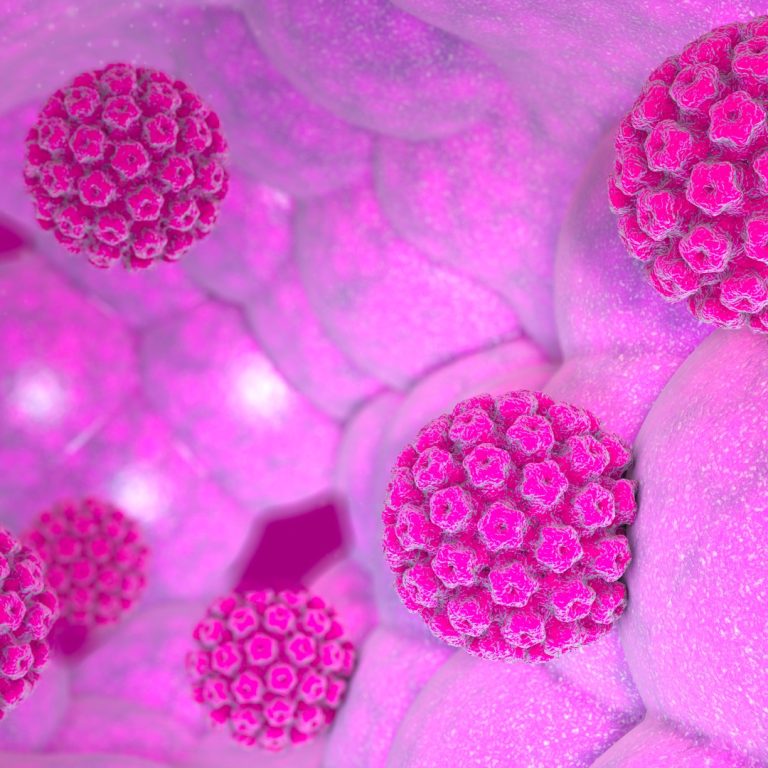
On Mar. 17, 1999, the Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the U.S….
In 1999, American Association for Cancer Research (AACR) Scientist-Survivor Program which united researchers with patients and survivor advocates…

In 1999, the Fred Hutchinson Business Alliance announced that the 1999 E. Donnall Thomas Medal of Achievement would…
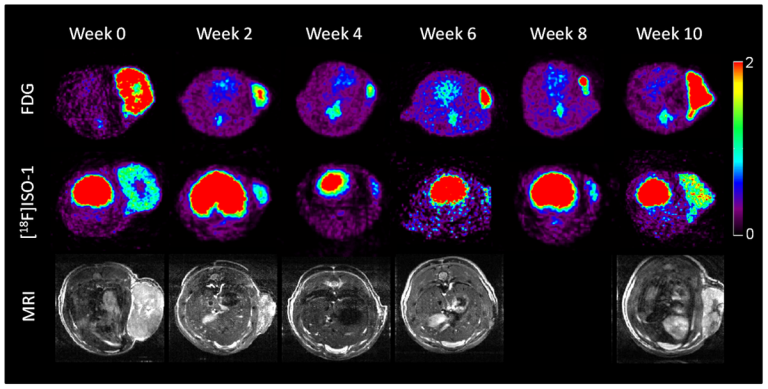
Dec. 22, 1998, researchers from Barnes-Jewish Hospital in St. Louis announced using a revolutionary system that uses superconducting…

On Nov. 26, 1998, Wisconsin biologist James Thomson reported the first isolation and culturing of human embryonic stem…

In Oct. 1998, the Fetal Alcohol Syndrome Surveillance Network was created to determine the prevalence of FAS within…

On Mar. 27, 1998. the Pfizer drug Viagra (Sildenafil) was approved by the FDA to treat erectile dysfunction….

Feb. 13, 1998, Dr. David Satcher was sworn in as U.S. Surgeon General.

In 1998, The Oregon College of Dentistry was founded in Portland.

In 1998, Medical College of Virginia surgeons performed country’s first living donor liver transplant between unrelated adults.

On Nov. 21, 1997, the FDA Modernization Act (FDAMA) was signed into law, amending the Food, Drug and…
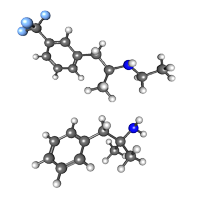
On Nov. 14, 1997, the U.S. Centers for Disease Control and Prevention (CDC) identified the link between fen-phen…

On Nov. 6, 1997, the National Cancer Institute Director’s Consumer Liaison Group was established to advise and provide…

On Sept. 15, 1997, Wyeth-Ayerst Laboratories and Interneuron Pharmaceuticals, manufacturers of the popular off-label obesity combination therapy known…
On Mar. 4, 1997, President Clinton issued an executive order that banned federal funds for cloning experiments. This…
In March 1997, the American Cancer Society (ACS) updated its recommended mammography screening interval for women ages 40-49…

On Feb. 13, 1997, the American Association for the Advancement of Science (AAAS) Annual Meeting and Science Innovation…