Moderna announced rolling submission of Biologics License Application with FDA for COVID-19 vaccine
On Jun. 1, 2021, Moderna announced that it had initiated the rolling submission process with the U.S. Food…

On Jun. 1, 2021, Moderna announced that it had initiated the rolling submission process with the U.S. Food…

On Jun. 1, 2021, Moderna announced that it had entered into an agreement with Thermo Fisher Scientific for…

On Jun. 1, 2021, CytoDyn announced that Chiral Pharma in the Philippines placed its first purchase order for…

On Jun. 1, 2021, the U.S. Department of Health and Human Services (HHS) unveiled a new type of…

On May 28, 2021, Amgen announced that the U.S. Food and Drug Administration (FDA) had approved LUMAKRAS (sotorasib)…

On May 28, 2021, Pfizer and BioNTech announced that the Conditional Marketing Authorization for COMIRNATY in the European…

On May 28, 2021, the Oregon Department of Agriculture (ODA) lifted the quarantine on the Oregon mink farm…

On May 27, 2021, Innovation Pharma announced that patient enrollment in the Company’s 120-patient, Phase 2 clinical trial…
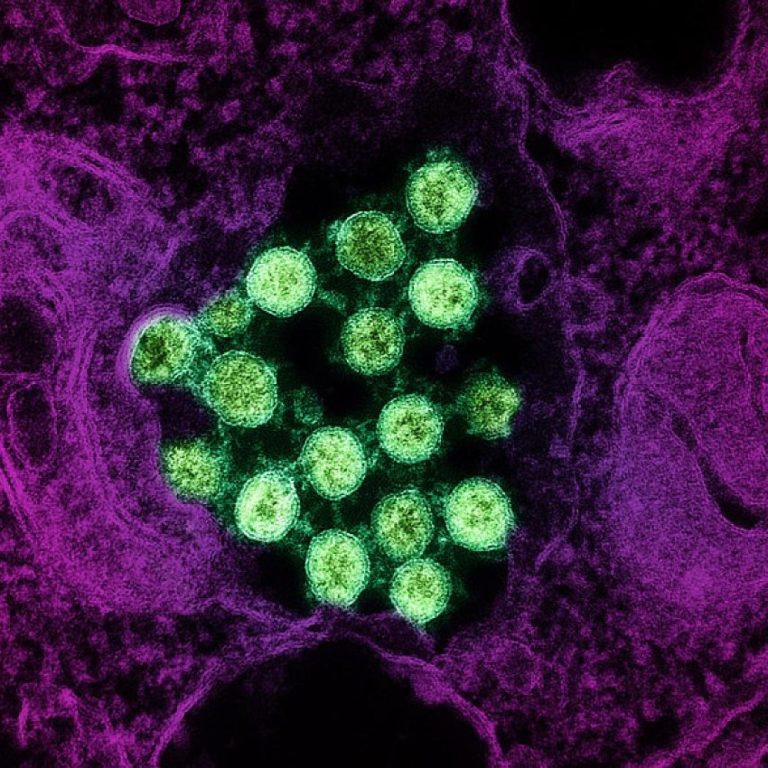
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…
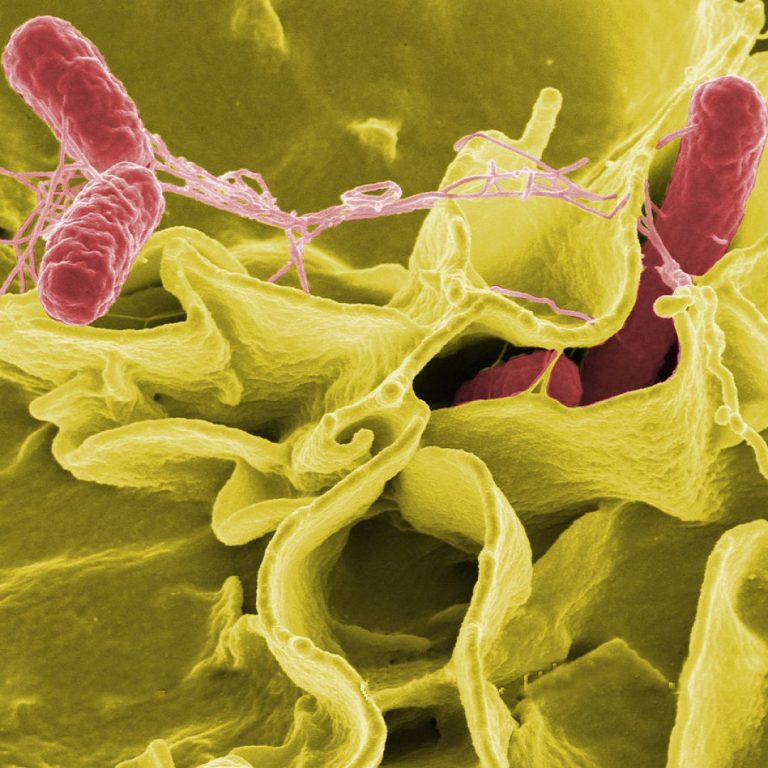
On May 26, 2021, National Institutes of Health (NIH) scientists reported that the immune system’s attempt to eliminate…
On May 25, 2021, Moderna announced that the Phase 2/3 study of its COVID-19 vaccine (mRNA-1273) in adolescents…
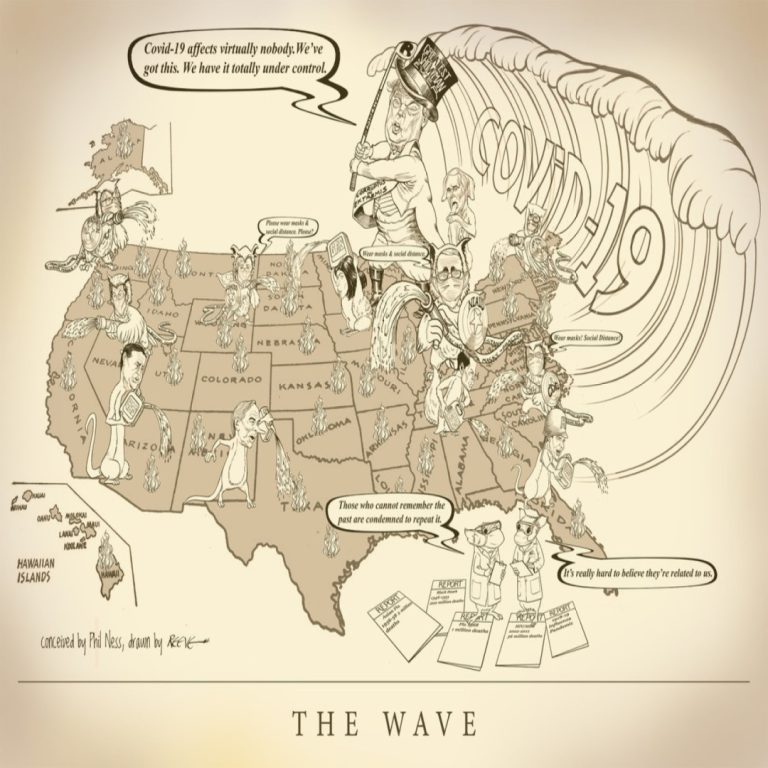
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…

On May 24, 2021, the World Health Organization (WHO) and the Swiss Confederation signed a Memorandum of Understanding…

On May 24, 2021, Quidel announced that its Sofia’ SARS Antigen FIA was the first rapid antigen test…
On May 24, 2021, Moderna and Aldevron announced their expanded collaboration in support of the Moderna COVID-19 Vaccine…

On May 22, 2021, Moderna and Samsung Biologics announced a Manufacturing Services and Supply Agreement in which Samsung…

On May 22, 2021, Novavax announced the signing of a non-binding memorandum of understanding (MoU) with the Ministry…

On May 21, 2021, the the U.S. Food and Drug Administration (FDA) approved Rybrevant (amivantamab-vmjw) as the first…

On May 21, 2021, Moderna announced that the Ministry of Health, Labour and Welfare (MHLW) of Japan granted…

On May 21, 2021, Moderna announced that the Ministry of Food and Drug Safety of South Korea (MFDS)…

On May 21, 2021, Novavax announced that the full results from the Phase 3, randomized, observer-blinded, placebo-controlled trial…

On May 21, 2021, Novavax announced participation in a mix-and-match (vaccine interchangeability) COVID-19 vaccine booster trial that led…

On May 20, 2021, Pfizer and BioNTech announced a new agreement with the European Commission (EC) to supply…

On May 19, 2021, Megan A. Cooper, MD, PhD, an associate professor of pediatrics at Washington University School…

On May 13, 2021, CureVac announced together with its collaboration partner GSK, first preclinical data in a rat…

On May 12, 2021, the province of British Columbia provided the ALS Society of BC with $2 million…

On May 12, 2021, Moderna announced a new supply agreement with the government of Australia for 25 million…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 10, 2021, the National Institutes of Health reported that an investigational gene therapy can safely restore…