U.S. Preventive Services Task Force recommended all women get screened for breast cancer every other year aged 40 to 74
On Apr. 30, 2024, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…

On Apr. 30, 2024, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
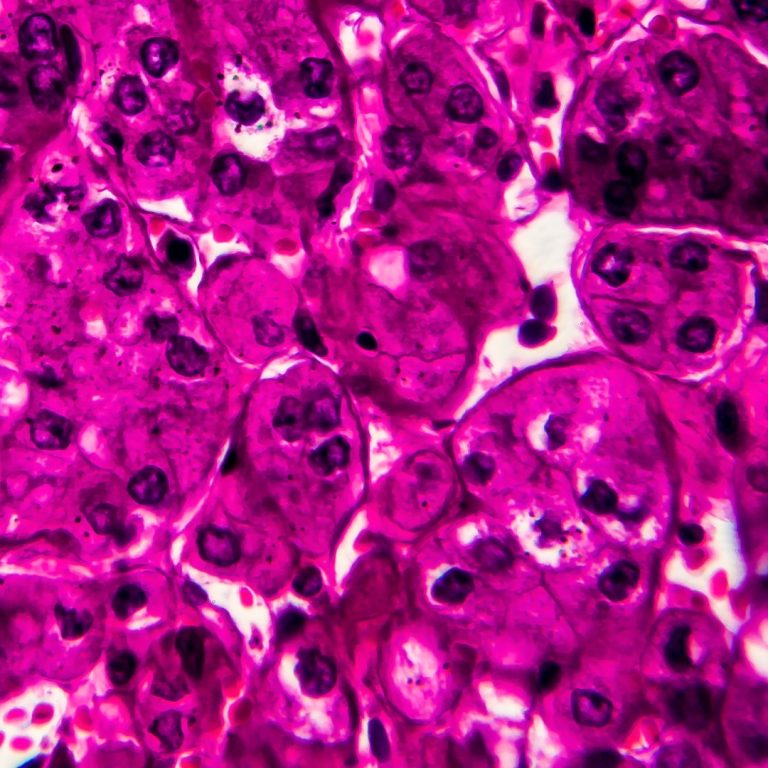
On Apr. 26, 2024, an international team of researchers led by the National Cancer Institute (NCI) released an…

On Mar. 14, 2024, Microsoft and the Fred Hutch announced a collaboration to end cancer faster, applying machine…

On Mar. 13, 2024, the Fred Hutchinson Cancer Center announced that a blood test intended for screening for…
On Feb. 21, 2024, the Fred Hutchinson Cancer Center announced it was at the helm of a new…

On Feb. 21, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved $98,592,155…

On Jan. 9, 2024, the Institute for Health Metrics and Evaluation (HHMI) reported that despite a gradual decline…
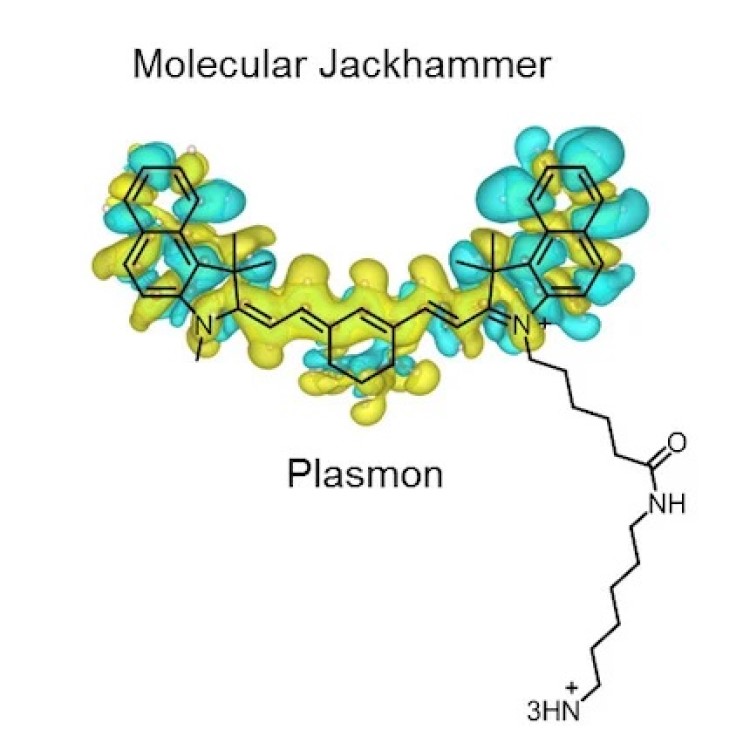
On Dec. 19, 2023, a research team led by Rice University reported they found that the atoms of…

On Dec. 14, 2023, Pfizer announced it had completed the acquisition of Seagen, a global biotechnology company based…
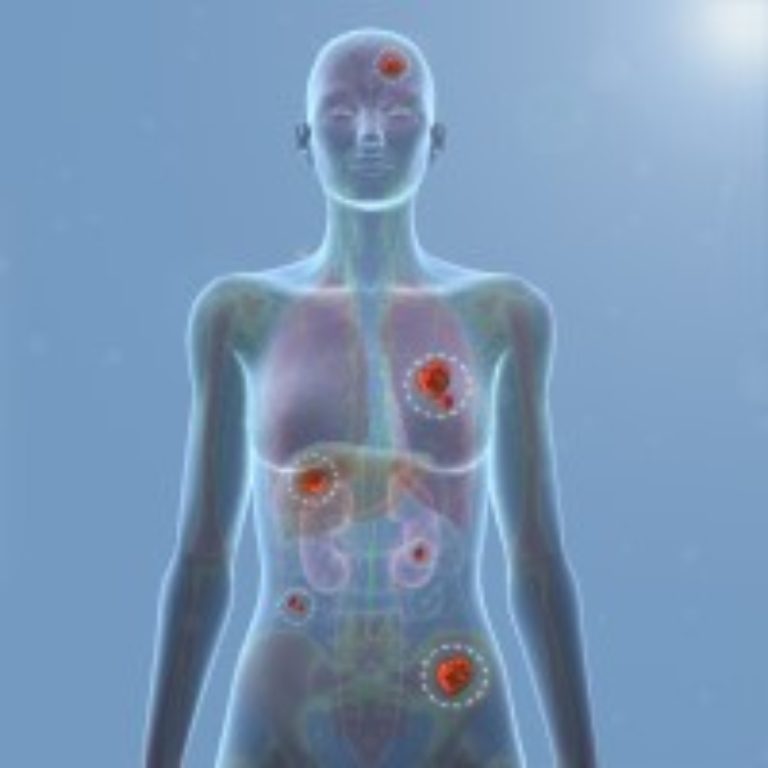
On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…

On Nov. 30, 2023, in a momentous landmark for medical research, UK Biobank announced that it had unveiled…

On Nov. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov. 13, 2023, the White House announced the first-ever Initiative on Womenメs Health Research, an effort led…

On Oct. 14, 2023, Purdue University ensured that the memory of the former graduate and devoted Boilermaker football…

On Oct. 7, 2023, California became the first state to ban four chemicals used in well-known candies and…
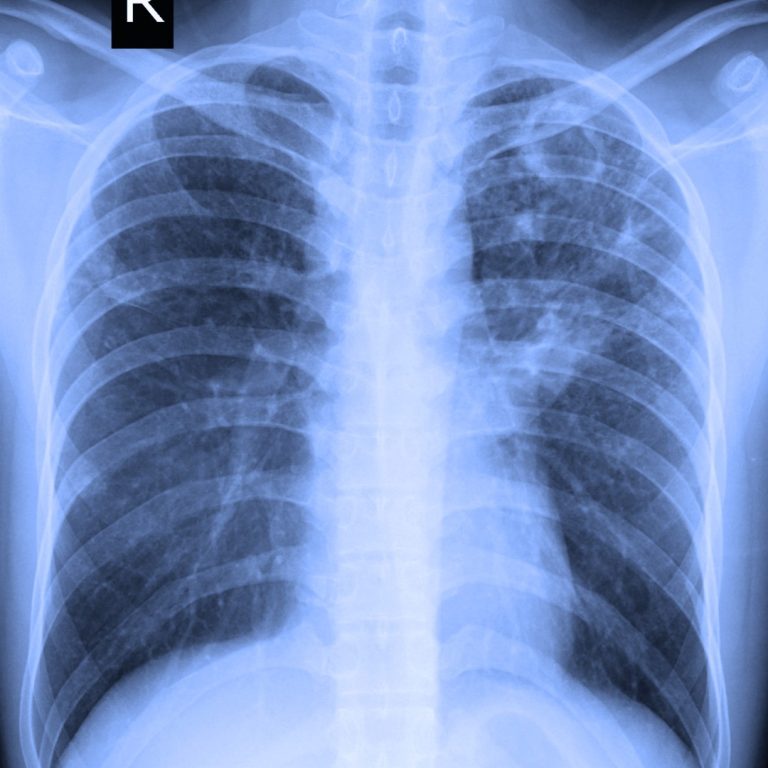
On Sept. 27, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that new diagnoses of…

On Sept. 26, 2023, the Advanced Research Projects Agency for Health (ARPA-H), an agency within the U.S. Department…

On Sept. 11, 2023, researchers at the National Institutes of Health (NIH) reported that living in an area…

On Sept. 6, 2023, the Markey Cancer Center (MCC) became Kentucky’s first and only National Cancer Institute (NCI)-Designated…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…
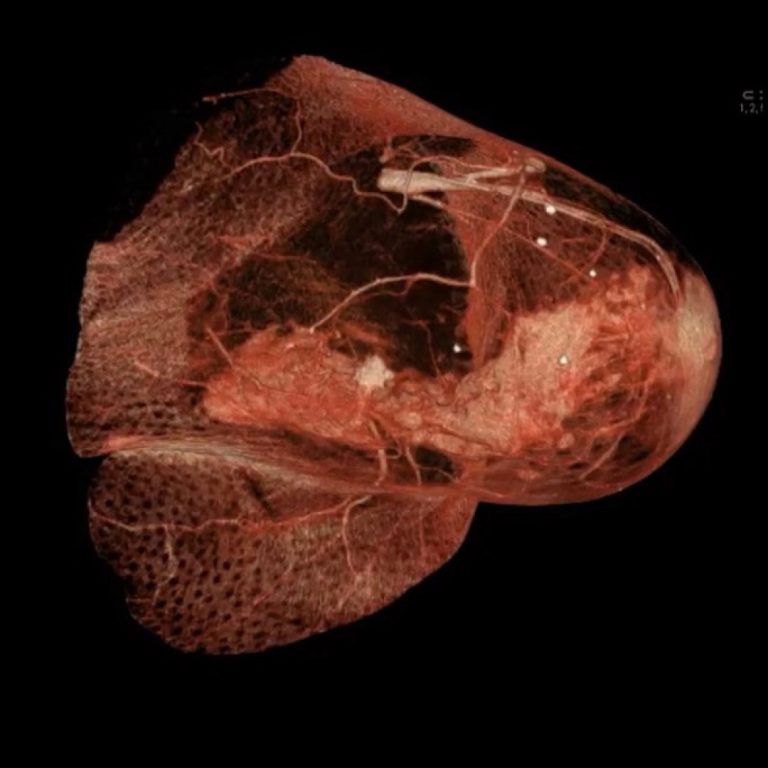
On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
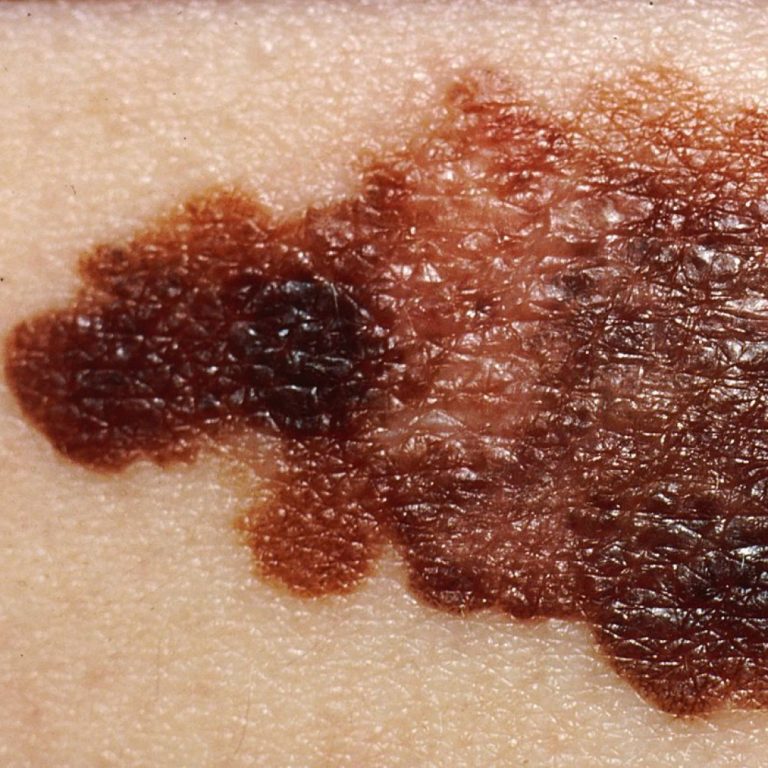
On May 1, 2023, an Oregon Health Sciences University (OHSU) dermatologist and a multi-disciplinary team confirmed that a…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…
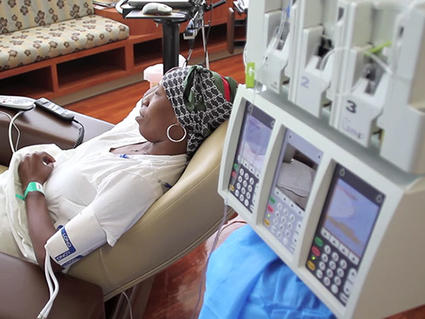
On Mar. 23, 2023, the Fred Hutchinson Cancer Center (FHCRC) announced a new building in South Lake Union…

On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…
On Feb. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a major step forward…

On Feb. 3, 2023, the World Health Organization (WHO) released an updated Global Breast Cancer Initiative (GBCI) Framework…

On Jan. 6, 2023, the Purdue Center for Cancer Research announced it was changing its name to the…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…