U.S. Food and Drug Administration approved 50 new therapeutics in 2024
On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…

On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…

On Dec. 10, 2024, a research team led by Fangqiong Ling, an assistant professor at Washington University in…

On Nov. 8, 2024, as companies begin considering moving their manufacturing operations outside of China in order to…

On Oct. 31, 2024, researchers from the University of Pittsburgh reported results of a study that showed drug-related…
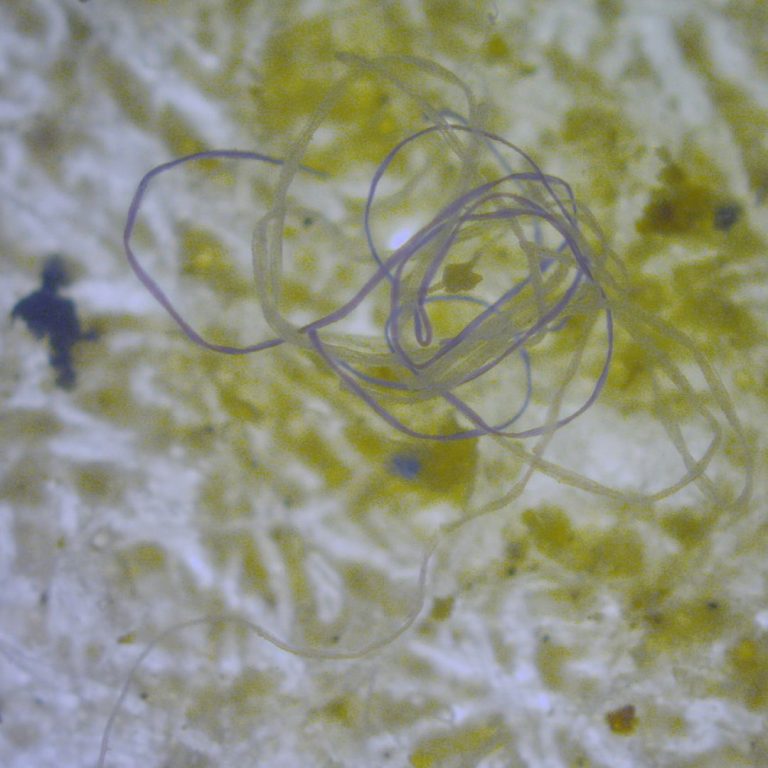
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…

On Oct. 16, 2024, Sanofi announced it will contribute $18 million to three Historically Black Medical Schools to…

On Oct. 14, 2024, Lundbeck and Longboard Pharmaceuticals announced an agreement for Lundbeck to acquire Longboard for payment…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…

On Oct. 2, 2024, Eli Lilly and Company announced a $4.5 billion investment to create the Lilly Medicine…

On Oct. 1, 2024, the American Cancer Society (ACS) released Breast Cancer Statistics, 2024, the organization’s biennial update…

On Sept. 17, 2024, researchers from Tufts University announced they had received a $20.7 million grant from the…

On Sep. 9, 2024, Santo Fortunato, a professor at Luddy School of Informatics, reported that an international research…
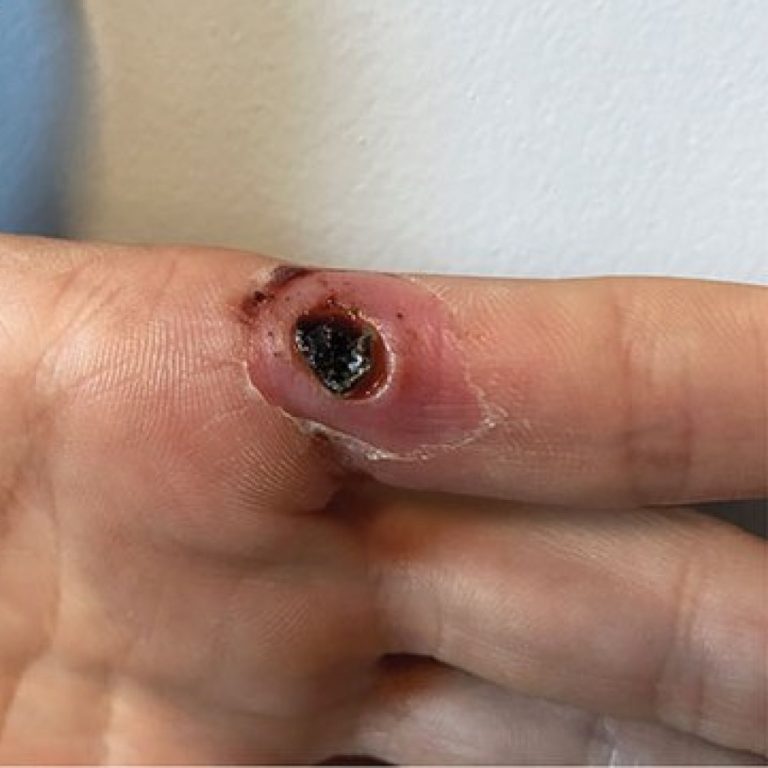
On Aug. 29, 2024, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Aug. 13, 2024, Eli Lilly announced the opening of the Lilly Seaport Innovation Center (LSC), a research…
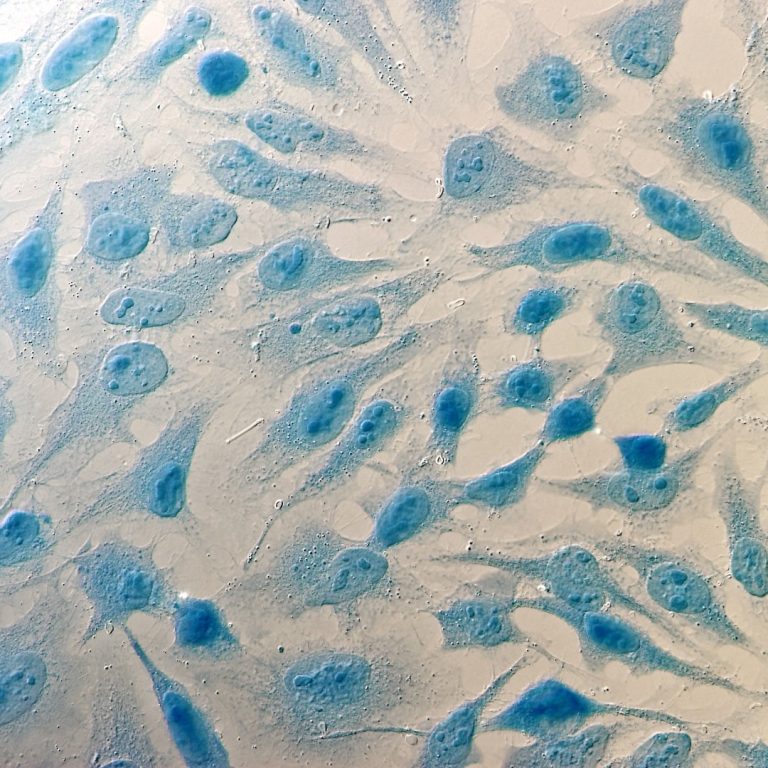
On Aug. 5, 2024, Novartis and Viatris were named a federal lawsuit in Maryland by the family of…
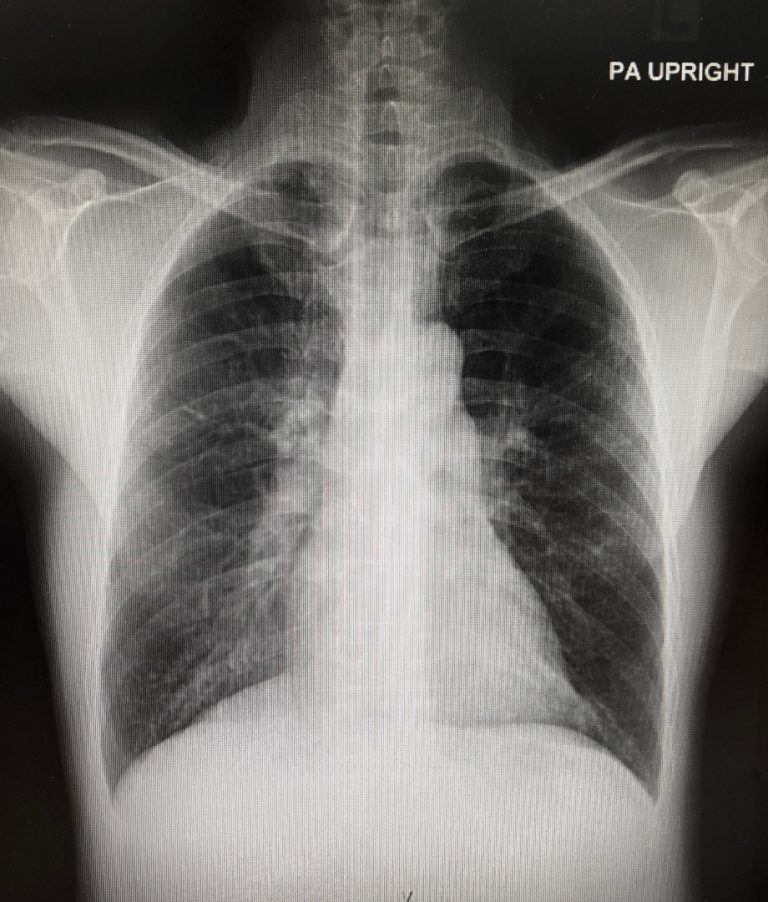
On Jul. 3, 2024, Regeneron Pharmaceuticals and Sanofi announced that the European Commission (EC) has approved Dupixent® (dupilumab)…
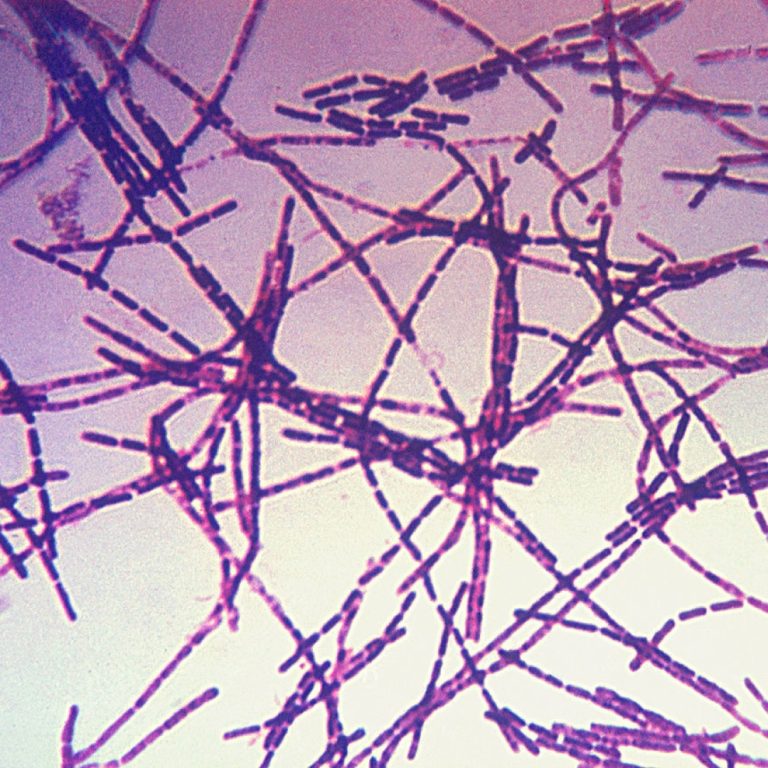
On Jul. 2, 2024, Emergent BioSolutions announced it had received more than $250 millionin contract modifications from the…

On Jun. 24, 2024, Novo Nordisk announced plans to invest 4.1 billion US dollars to build a second…

On Jun. 17, 2024, Merck announced that the U.S. Food and Drug Administration (FDA) had approved CAPVAXIVE™ (Pneumococcal…

On Jun. 13, 2024, BioMADE announced an investment in a state-of-the-art bioindustrial manufacturing infrastructure in Minnesota, following support…

Montana Life Science Genealogy illustrates life science companies and non-profit research organization in the State of Montana by…

On Jan. 5, 2024, the U.S. Food and Drug Administration (FDA) authorized Florida’s Agency for Health Care Administration’s…

On Dec. 14, 2023, Pfizer announced it had completed the acquisition of Seagen, a global biotechnology company based…
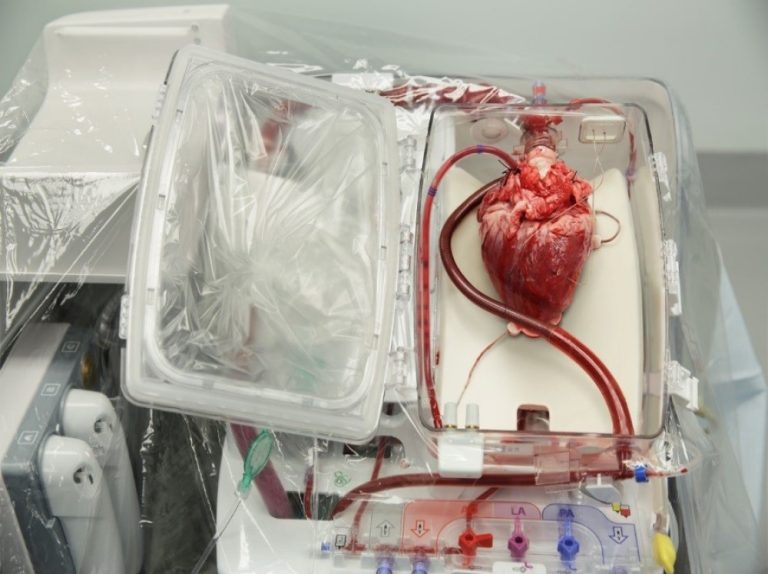
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…
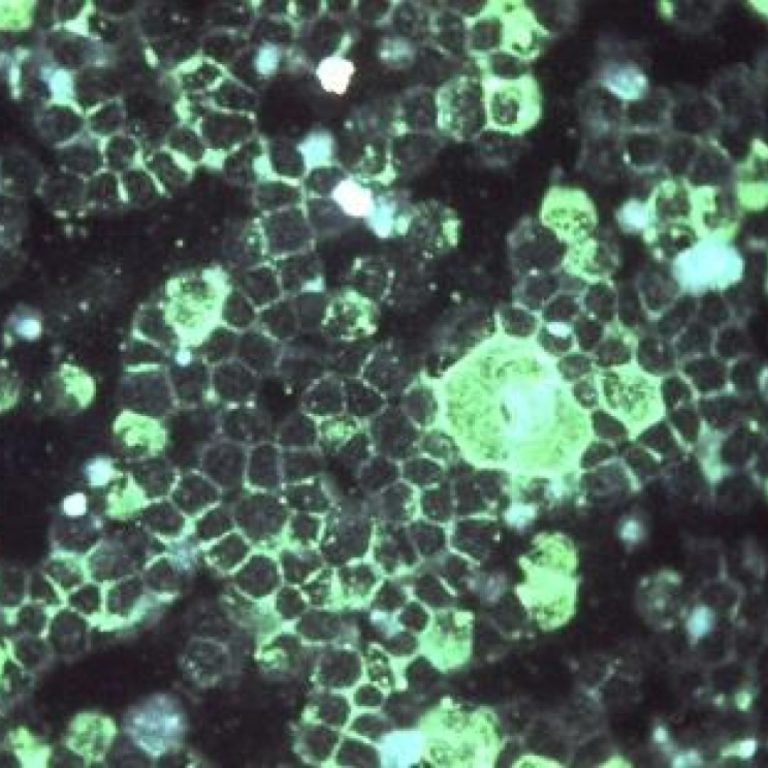
On May 31, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…
On Mar. 20, 2023, NIH scientists announced that the magnitude and quality of a key immune cellメs response…
On Mar. 14, 2023, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) of the…

On Mar. 13, 2023, Pfizer announced an agreement to acquire Seagen, a global biotechnology company that discovers, develops…

On Mar. 10, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ZAVZPRET (zavegepant),…