Diverse genome sequences provided a powerful tool for studying risk of heart disease
On Dec. 8, 2021, in a large-scale study of people from diverse ancestries, researchers narrowed down the number…

On Dec. 8, 2021, in a large-scale study of people from diverse ancestries, researchers narrowed down the number…
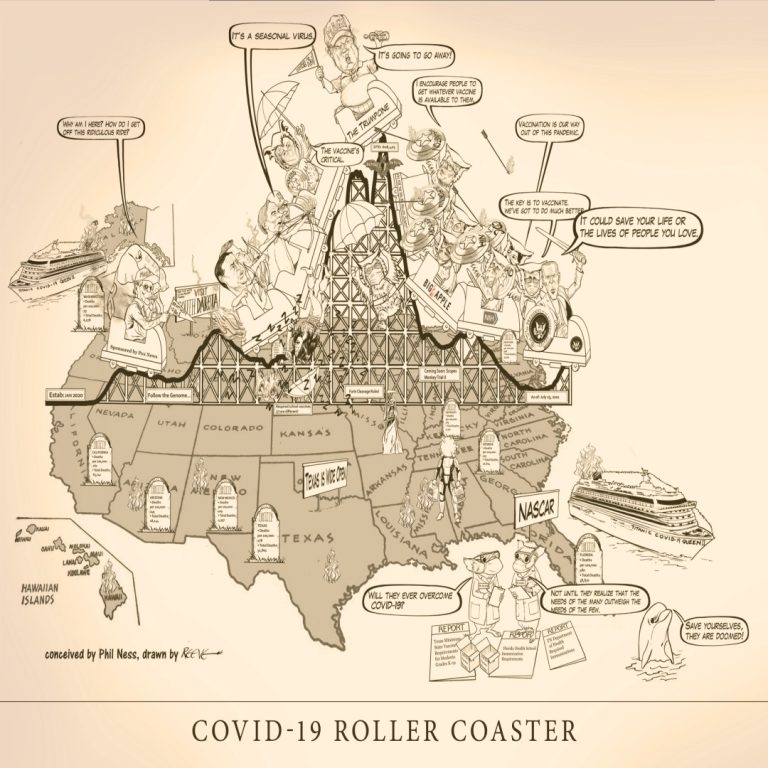
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…

On Oct. 21, 2021, Pfizer and BioNTech announced topline results from a Phase 3 randomized, controlled trial evaluating…

On Oct. 18, 2021, Pfizer and BioNTech announced that the European Medicines Agency’s Committee for Human Medicinal Products…
On Oct. 15, 2021, Pfizer and BioNTech announced they had submitted data supporting the vaccination of children 5…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Oct. 4, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…
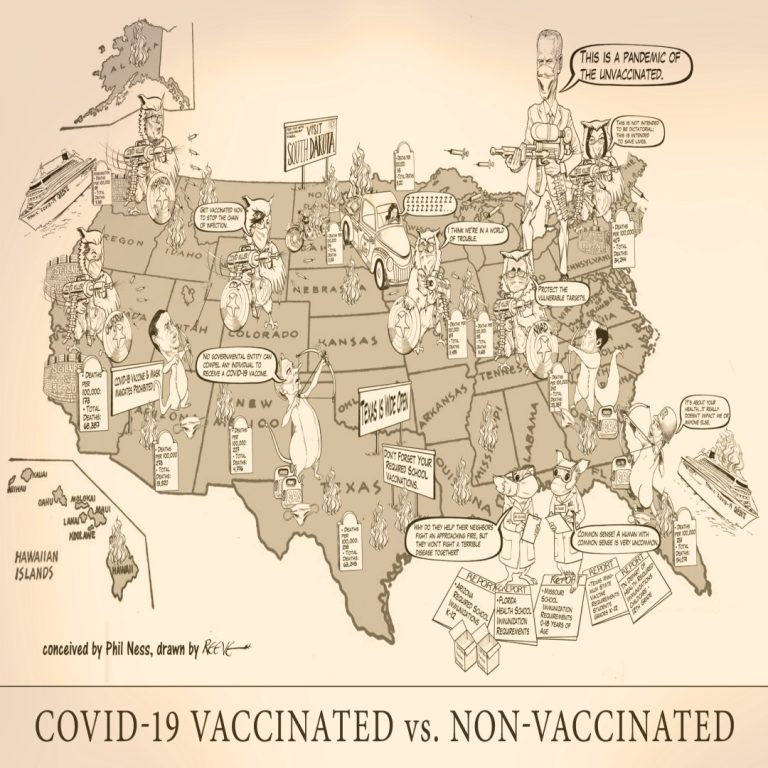
This cartoon illustrates the various issues facing individuals across the U.S. who are vaccinated against COVID-19 verus those…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 29, 2021, Roche confirmed positive data from the phase II/III 2066 study, investigating Ronapreveル (casirivimab and…

On Sept. 28, 2021, Sanofi announced that it had terminated plans for its own mRNA-based COVID-19 vaccine given…

On Sept. 14, 2021, Regeneron announced an agreement with the U.S. Department of Health and Human Services and…

On Sept. 6, 2021, Pfizer and BioNTech announced that they had submitted a variation to the European Medicines…

On Aug. 26, 2021, Inovio Pharma announced that it had received regulatory authorization from Brazil’s ANVISA (Agencia Nacional…

On Aug. 25, 2021, Pfizer and BioNTech announced the initiation of a supplemental Biologics License Application (sBLA) to…

On Aug. 23, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Jul. 29, 2021, Cocrystal Pharma announced that its SARS-CoV-2 3CL protease lead CDI-45205 and several analogs showed…
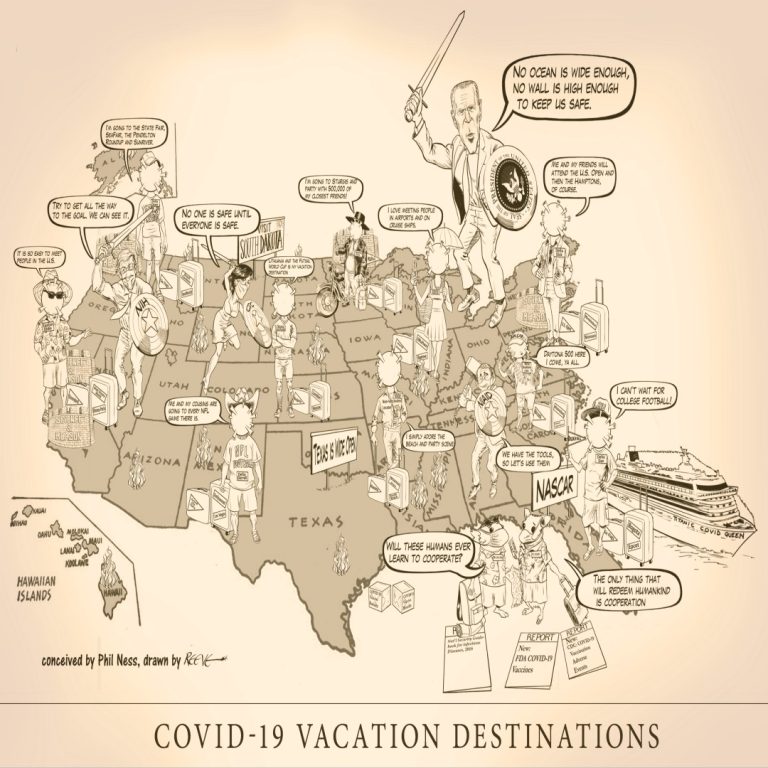
Planning a Summer vacation? Where to go? How about going to one of those large events with tens…
On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…

On Jun. 21, 2021, Cumberland Pharma released a series of case reports describing the effectiveness of Vibativ (telavancin)…
On Jun. 14, 2021, Cocrystal Pharma announced the selection of CDI-45205 as the lead compound for further development…

On Jun. 8, 2021, the University of Oxford announced that the RECOVERY trial found aspirin does not improve…

On May 27, 2021, Innovation Pharma announced that patient enrollment in the Company’s 120-patient, Phase 2 clinical trial…

On May 20, 2021, Pfizer and BioNTech announced a new agreement with the European Commission (EC) to supply…
On Apr. 29, 2021, Pfizer and BioNTech announced they had submitted a variation to the Conditional Marketing Authorization…

COVID-19 and it’s naysayers are attacking Science and Reason. The defenders must suffer the slings and arrows from…