IHME reported global diabetes cases to soar from 529 million to 1.3 billion by 2050
On Jun. 22, 2023, the Institute for Health Metrics and Evaluation (IHME) reported that more than half a…

On Jun. 22, 2023, the Institute for Health Metrics and Evaluation (IHME) reported that more than half a…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On Jun. 17, 2023, the Mississippi man known as “Case 1,” the first person to be diagnosed with…

Milestones in Parkinson’s illustrates advancements in the disease from James Parkinson’s essay on ‘shaking palsy’ to development of…
On May 31, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…

On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved the oral antiviral Paxlovid (nirmatrelvir tablets…

On May 24, 2023, the U.S. FDA approved Braeburn pharmaceutical’s Brixadi (buprenorphine) extended-release injection for subcutaneous use (under…

On May 22, 2023, the Institute for Health Metrics and Evaluation (IHMI) reported low back pain remains the…
On May 19, 2023, the U.S. Food and Drug Administration (FDA) approved Krystal Biotech’s Vyjuvek, a herpes-simplex virus…

On May 11, 2023, the U.S. Food and Drug Administration (FDA) announced the supplemental approval of Otsuka Pharmaceutical…
On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
On May 8, 2023, scientists at the National Institutes of Health (NIH) announced that they had identified new…

On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…
On May 3, 2023, National Institutes of Health (NIH) scientists announced that they had uncovered new details on…
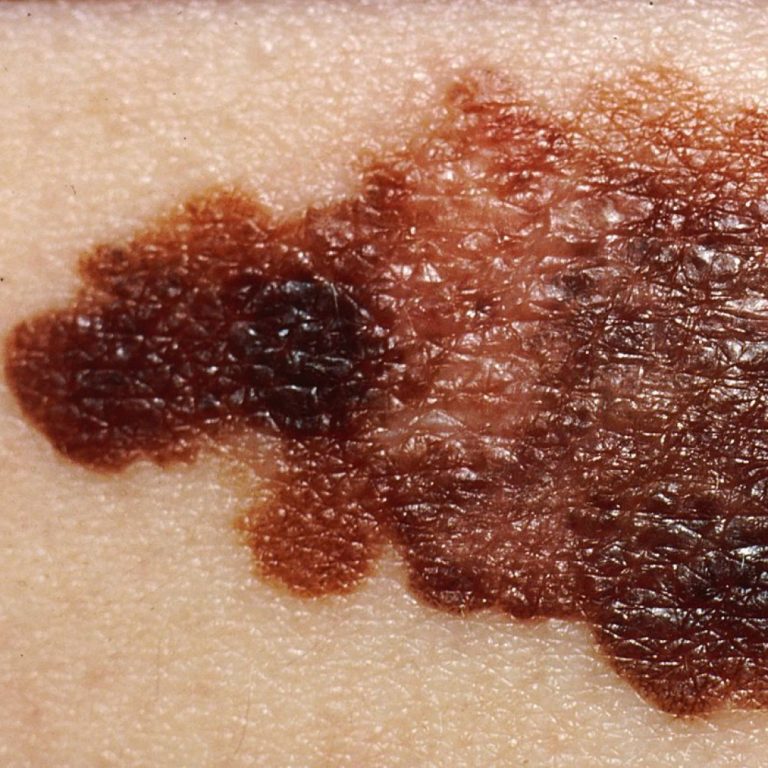
On May 1, 2023, an Oregon Health Sciences University (OHSU) dermatologist and a multi-disciplinary team confirmed that a…

On Apr. 27, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 27, 2023, the Zoonomia Project team announced in in a special issue of Science, that had…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 20, 2023, Massachusetts Institute of Technology (MIT) researchers announced that have developed machine-learning algorithms that can…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Apr. 17, 2023, a new initiative led by Jennifer Doudna and Jill Banfield at the Innovative Genomics…

On Apr. 14, 2023, Revance Therapeutics announced that the U.S. Food and Drug Administration (FDA) had approved the…

On Apr. 13, 2023, in an enormous leap forward in the understanding of Parkinson’s disease (PD), researchers announced…
On Apr. 10, 2023, the U.S. Department of Health and Human Services (HHS) announced it was spending over…

On Apr. 5, 2023, researchers at Oregon Health & Science University’s OHSU Casey Eye Institute announced that gene…
On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…
On Mar. 23, 2023, the Fred Hutchinson Cancer Center (FHCRC) announced a new building in South Lake Union…
On Mar. 20, 2023, some of the world’s leading makers of flu vaccines said they could make hundreds…

On Mar. 17, 2023, PrairiesCan announced $80,514,000 over five years to support the Canadian Critical Drug Initiative and…