FDA Advisory Committee voted in support of favorable benefit-risk profile for Pfizer’s PAXLOVID
On Mar. 16, 2023, Pfizer announced that the U.S. Food and Drug Administration’s Antimicrobial Drugs Advisory Committee (AMDAC)…

On Mar. 16, 2023, Pfizer announced that the U.S. Food and Drug Administration’s Antimicrobial Drugs Advisory Committee (AMDAC)…
On Mar. 15, 2023, scientists at Texas A&M announced that a research study: ‘RNA interference is essential to…
On Mar. 13, 2023, Mayo Clinic published a study that showed the COVID-19 pandemic was linked to a…

On Mar. 13, 2023, Pfizer announced an agreement to acquire Seagen, a global biotechnology company that discovers, develops…

On Mar. 10, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ZAVZPRET (zavegepant),…

On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…
On Mar. 8, 2023, Amphastar Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval…
On Feb. 28, 2023, Public Health Delta & Menominee Counties (PHDM) was alerted to three cases of atypical…

On Feb. 20, 2023, results from a randomized clinical trial of Ivermectin in a higher-hose and longer duration…
On Feb. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a major step forward…
On Feb. 15, 2023, it was reported that a 53-year-old man in Germany had become at least the…

On Feb. 14, 2023, the Pacific Economic Development Agency of Canada (PacifiCan), announced $14.5 million in funding for…
On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…

On Feb. 3, 2023, the World Health Organization (WHO) released an updated Global Breast Cancer Initiative (GBCI) Framework…
On Feb. 2, 2023, a National Institutes of Health research group with extensive experience studying ebolavirus countermeasures successfully…

On Jan. 31, 2023, a team of researchers led by the Innovation Center for Neurological Disorders and Department…

On Jan. 26, 2023, Twist Bioscience announced that it had begun offering high-quality synthetic DNA using its silicon…
On Jan. 25, 2023, it was reported that adults living in rural areas of the United States have…
On Jan. 25, 2023, researchers at Oregon Health & Science University (OHSU) reported that Immunity from COVID-19 appears…

Our Milestones in CRISPR cartoon illustrates several significant achievements in the development of the technology with commentary by…
On Jan. 10, 2023, the U.S. National Institutes of Health (NIH) reported that antiviral treatments can help reduce…

On Jan. 6, 2023, the Purdue Center for Cancer Research announced it was changing its name to the…
On Jan. 6, 2023, the U.S. Food and Drug Administration (FDA) approved Leqembi (lecanemab-irmb) via the Accelerated Approval…
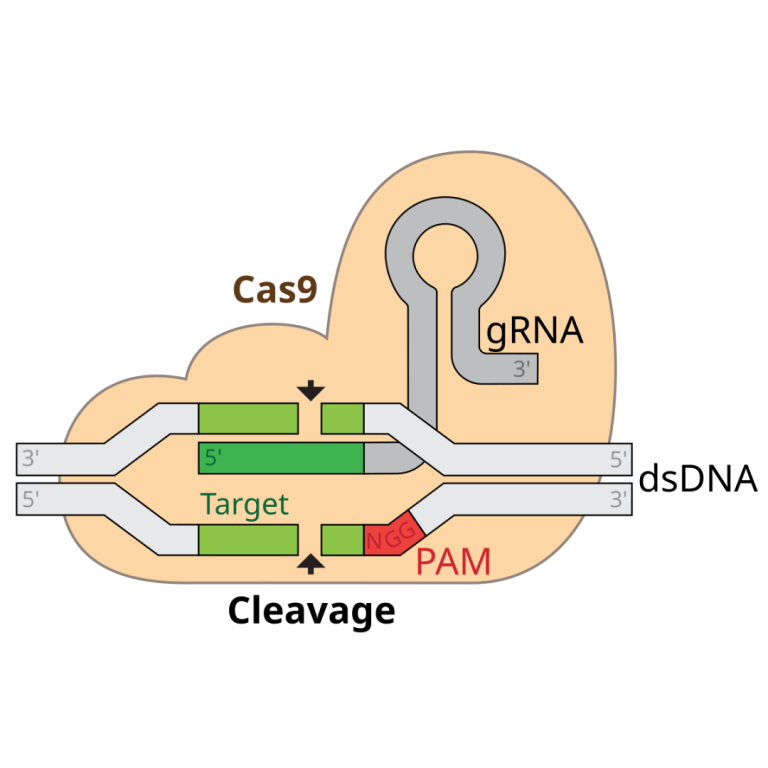
On Jan. 4, 2023, an international research group announced it had for the first time reconstructed ancestors dating…

Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…
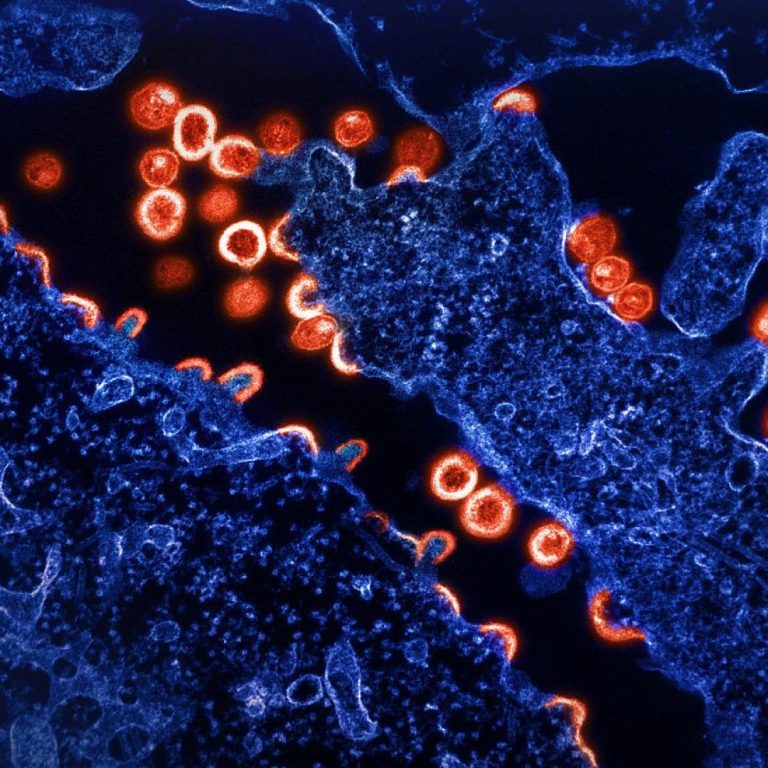
On Dec. 22, 2022, Gilead Sciences announced that Sunlenca (lenacapavir), in combination with other antiretroviral(s) (ARV), had been…

On Dec. 13, 2022, Texas Biomed announced that it had been designated as a prime contractor by the…

On Dec. 10, 2022, CSL announced data affirming the long-term durability and safety of single-infusion HEMGENIX® (etranacogene dezaparvovec-drlb)…

On Dec. 9, 2022, the World Health Organization (WHO) announced that the first doses of one of the…