HHS launched first venture capital partnership to develop transformative technologies to combat future pandemics
On Jun. 1, 2021, the U.S. Department of Health and Human Services (HHS) unveiled a new type of…

On Jun. 1, 2021, the U.S. Department of Health and Human Services (HHS) unveiled a new type of…

On May 28, 2021, Amgen announced that the U.S. Food and Drug Administration (FDA) had approved LUMAKRAS (sotorasib)…

On May 28, 2021, Pfizer and BioNTech announced that the Conditional Marketing Authorization for COMIRNATY in the European…

On May 27, 2021, Innovation Pharma announced that patient enrollment in the Company’s 120-patient, Phase 2 clinical trial…
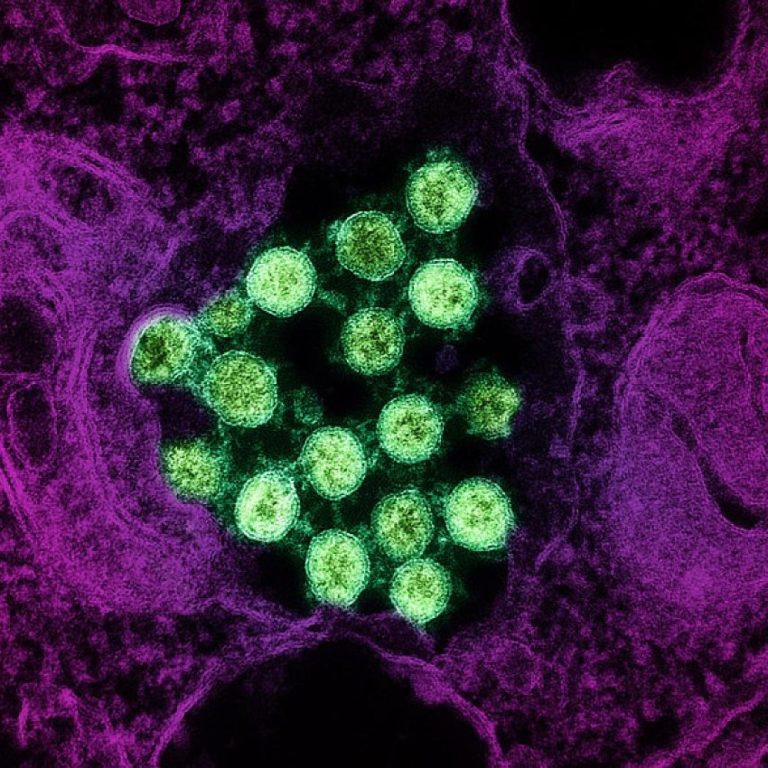
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…
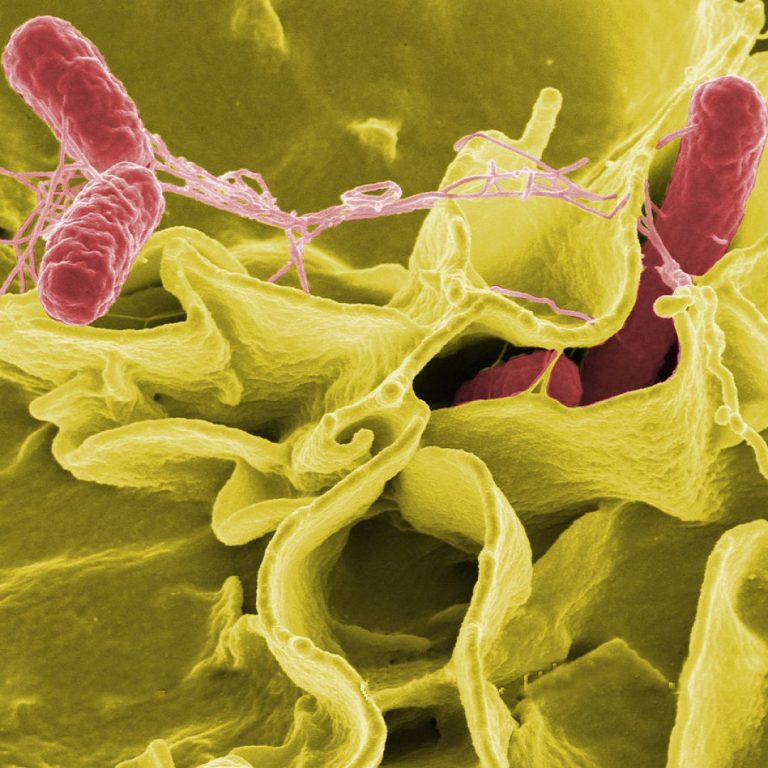
On May 26, 2021, National Institutes of Health (NIH) scientists reported that the immune system’s attempt to eliminate…
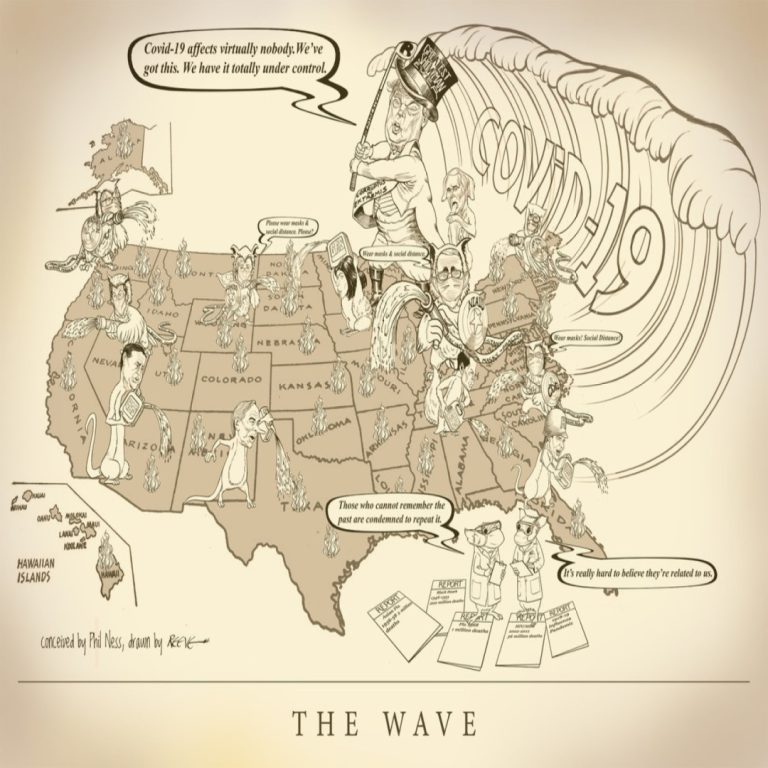
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…
On May 24, 2021, Moderna and Aldevron announced their expanded collaboration in support of the Moderna COVID-19 Vaccine…

On May 21, 2021, the the U.S. Food and Drug Administration (FDA) approved Rybrevant (amivantamab-vmjw) as the first…

On May 20, 2021, Pfizer and BioNTech announced a new agreement with the European Commission (EC) to supply…

On May 19, 2021, Megan A. Cooper, MD, PhD, an associate professor of pediatrics at Washington University School…

On May 13, 2021, CureVac announced together with its collaboration partner GSK, first preclinical data in a rat…

On May 12, 2021, the province of British Columbia provided the ALS Society of BC with $2 million…
On May 5, 2021, Moderna announced initial data from its Phase 2 study showing that a single 50…

On May 5, 2021, Hoth Therapeutics announced it has expanded its sponsored research agreement with Virginia Commonwealth University…

On May 5, 2021, CytoDyn announced an agreement to partner with the Albert Einstein Israelite Hospital (AEIH) in…
On Apr. 29, 2021, Pfizer and BioNTech announced they had submitted a variation to the Conditional Marketing Authorization…

COVID-19 and it’s naysayers are attacking Science and Reason. The defenders must suffer the slings and arrows from…

On Apr. 26, 2021, Moderna announced that it had entered into an agreement with Sanofi for fill/finish sterile…

On Apr. 23, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced that it had awarded…
On Apr. 19, 2021, Pfizer and BioNTech announced supplying an additional 100 million doses of COMIRNATY, the companies…

On Apr. 19, 2021, the Broad Institute of MIT in partnership with the Centers for Disease Control and…

On Apr. 19, 2021, the Mayo Clinic announced a study that bolstered evidence that colorectal cancer is often…

On Apr. 19, 2021, Tonix Pharmaceuticals and OyaGen announced an exclusive worldwide licensing agreement for an antiviral inhibitor…

On Apr. 19, 2021, scientists at the Texas A&M University Global Health Research Complex (GHRC) announced they had…

On Apr. 19, 2021, CytoDyn announced it had submitted the manufacturing section (CMC) of the application for an…
On Apr. 15. 2021, a team of researchers led by the University of Minnesota Medical School announced they…

On Apr. 15, 2021, the World Health Organization (WHO) reported that the world was still failing to develop…

On Apr. 15, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had had established…

On Apr. 15, 2021, CytoDyn announced it had executed an exclusive supply and distribution agreement with Chiral Pharma…