La Jolla Institute for Immunology awarded $6.4 million for international efforts to beat COVID-19
On Aug. 20, 2020, the La Jolla Institute for Immunology (LJI) announced that Erica Ollmann Saphire, Ph.D. had…
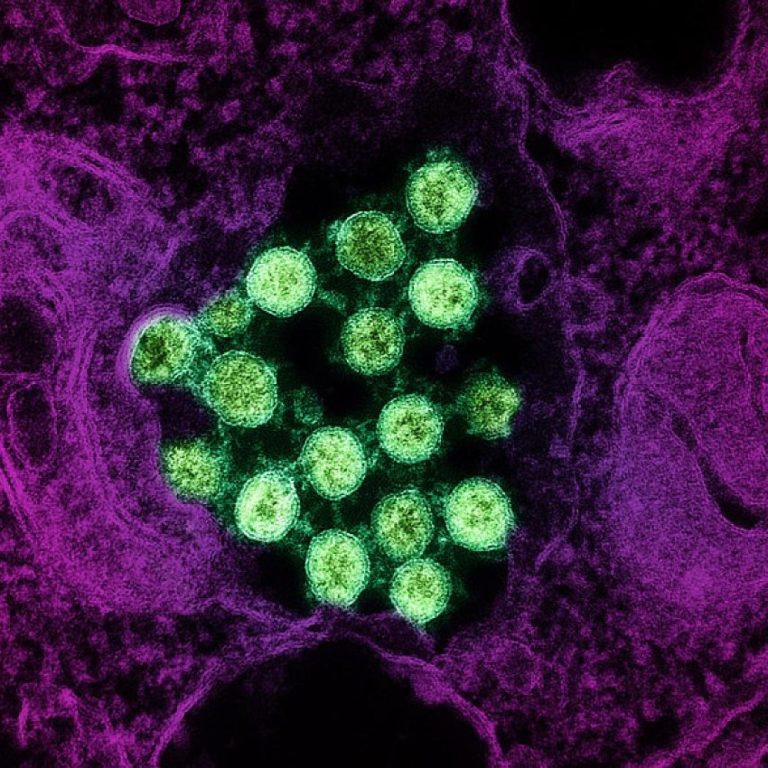
On Aug. 20, 2020, the La Jolla Institute for Immunology (LJI) announced that Erica Ollmann Saphire, Ph.D. had…
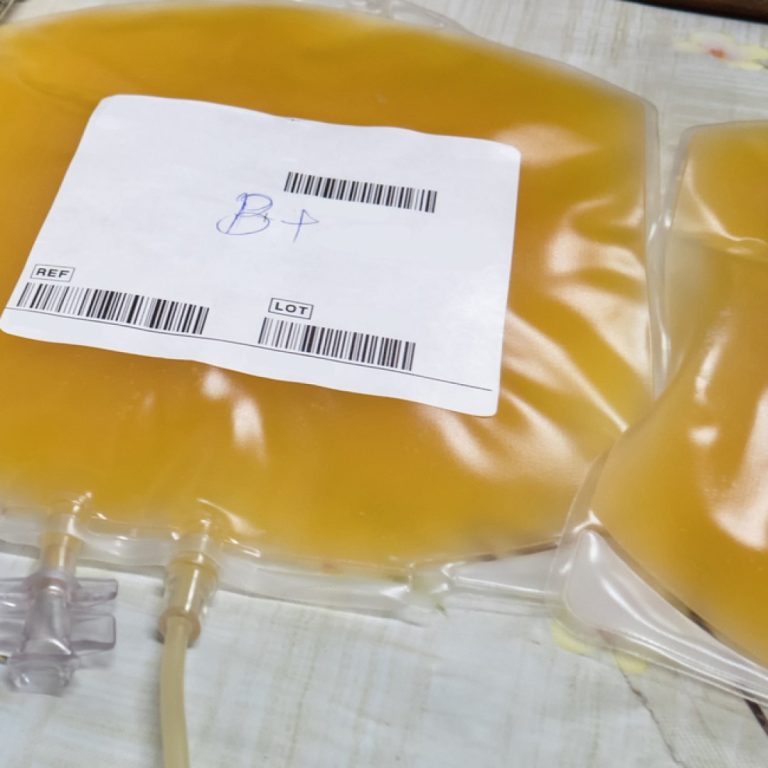
On Aug. 17, 2020, Scientisfic American reported that Costa Rican scientists at the Clodomiro Picado Institute have inoculated…

On Aug. 17, 2020, XBiotech announced that it had identified True Human antibodies that could potentially be used…
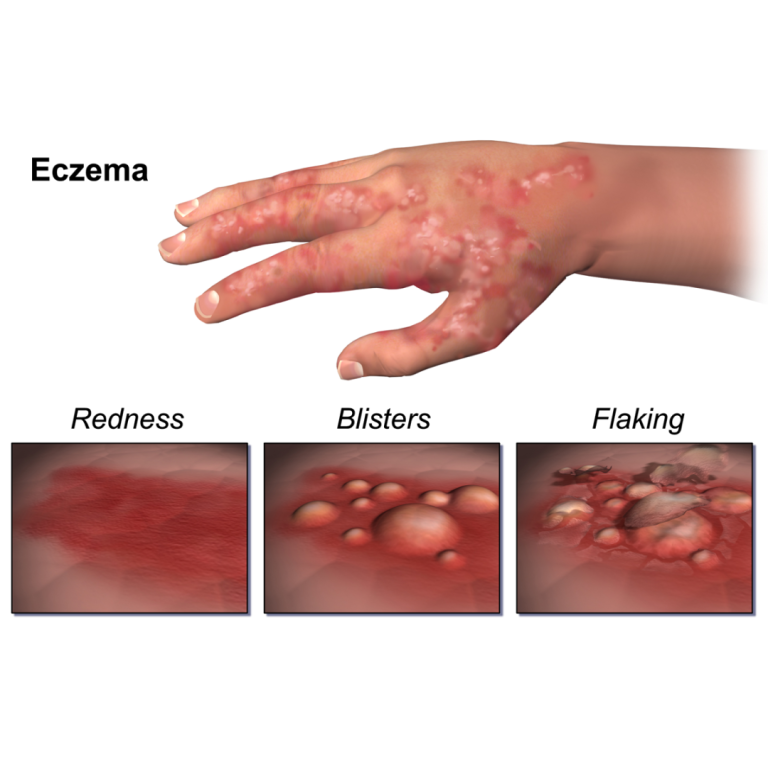
On Aug. 14, 2020, research supported by the National Institutes of Health delineated how two relatively common variations…

On Aug. 14, 2020, Medigen Vaccine Biologics (MBV) published preclinical results of their COVID-19 vaccine candidate in BioRxiv,…

On Aug. 13, 2020, in a first-of-its-kind study in humans, investigators at the Center for ADHD Research at…
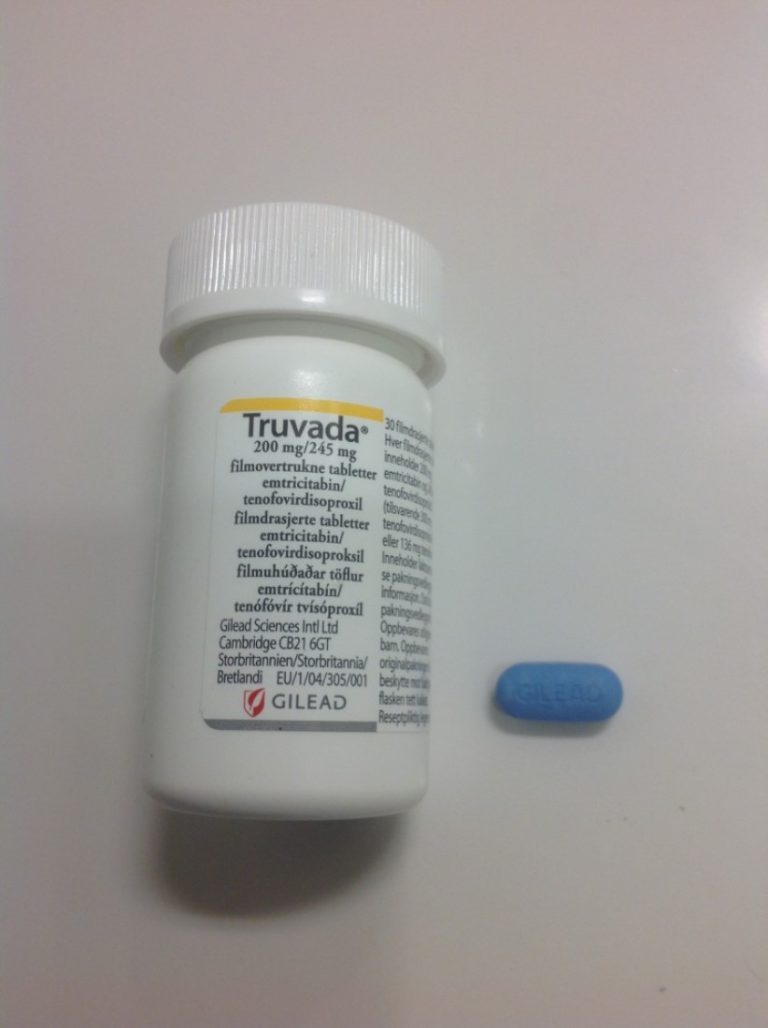
On Aug. 11, 2020, Gilead Sciences announced that the China National Medical Products Administration (NMPA) had approved a…
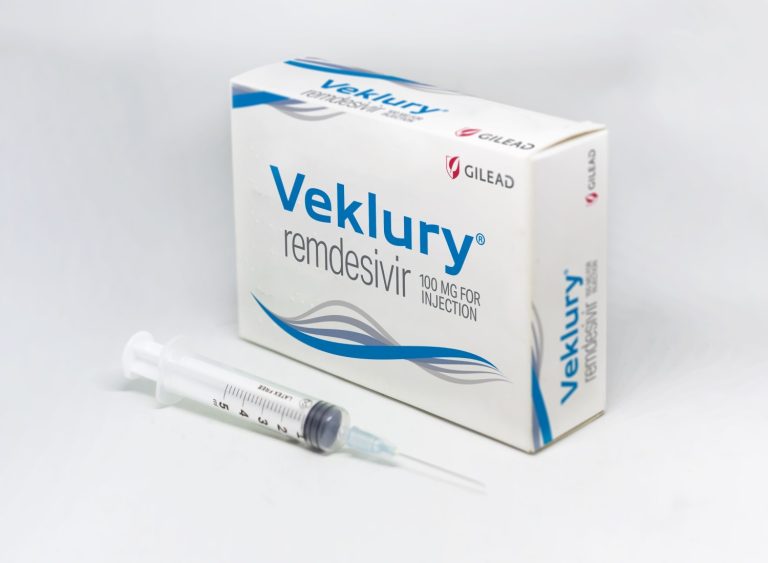
On Aug. 10, 2020, Gilead Sciences announced that it had submitted a New Drug Application (NDA) to the…

On Aug. 7, 2020, the U.S. Food and Drug Administration (FDA) approved Genentech’s Evrysdi (risdiplam) to treat patients…

On Aug. 6, 2020, RedHill Biopharma announced approval from the Mexican Federal Committee for the Protection against Sanitary…

On Aug. 6, 2020, Novavax announced it had entered a supply and license agreement with the Serum Institute…

On Aug. 4, 2020, Mateon Therapeutics and Abiogenesis announced they had initiated a ARTI-19 randomized, controlled, multi-site India…

On Aug. 4, 2020, Cocrystal Pharma announced the publication of preclinical animal studies of coronavirus antiviral compounds in…

On Jul. 31, 2020, the Centers for Disease Control and Prevention (CDC) awarded $109 million to state and…
On Jul. 31, 2020, researchers from the Pacific Northwest National Laboratory (PNNL) and Oregon Health & Science University…

On Jul. 30, 2020, RedHill Biopharma announced that it has initiated a global Phase 2/3 clinical study evaluating…

On Jul. 29, 2020, the Encyclopedia of DNA Elements (ENCODE) Project is a worldwide effort to understand how…
On Jul. 29, 2020, Regeneron announced that the Biomedical Advanced Research and Development Authority (BARDA) had entered into…
On Jul. 28, 2020, Soligenix announced publication of pre-clinical immunogenicity studies for its CiVax program (heat stable COVID-19…
On Jul. 28, 2020, Soligenix announced publication of pre-clinical immunogenicity studies for its CiVax™ program (heat stable COVID-19…

On Jul. 27, 2020, Humanigen announced that the National Institute of Allergy and Infectious Diseases and Humanigen had…

On Jul. 27, 2020, Immunic announced enrollment of the first patients in an investigator-sponsored phase 2 clinical trial…
On Jul. 27, 2020, Onconova Therapeutics announced that it had submitted an application with the National Institute of…

On Jul. 23, 2020, Dynavax Technologies and Medigen Vaccine Biologics (MVC) announced a collaboration to develop an adjuvanted…
On Jul. 22, 20120, Evotec announced that the U.S. Department of Defense (DoD) had awarded its Seattle-based subsidiary,…

On Jul. 22, 2020, RedHill Biopharma announced that it had submitted a Clinical Trial Application (CTA) with the…
On Jul. 20, 2020, Sorrento announced that its partner Mabpharm had received approval of its New Drug Application…

On Jul. 20, 2020, Mateon Therapeutics announced the launch of its global observational study called ARTI-19, for Artemisinin…

On Jul. 16, 2020, RedHill Biopharma announced approval from the Ministry of Health of the Russian Federation for…

On Jul. 16, 2020, Tonix Pharmaceuticals announced it had entered into a research collaboration and option agreement with…