Two fatal human influenza A/H5N1 (Bird Flu) virus infections reported in Cambodia
On Oct. 12, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that two fatal human…

On Oct. 12, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that two fatal human…

On Oct. 11, 2023, the European Commission, the European Investment Bank and the Bill & Melinda Gates Foundation…
On Oct. 9, 2023, GlaxoSmithKline announced that it had reached an exclusive agreement with Chongqing Zhifei Biological Products…

On Oct. 3, 2023, Vir Biotechnology announced that the Biomedical Advanced Research and Development Authority (BARDA), part of…

On Oct. 3, 2023, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of…
On Oct. 3, 2023, Novavax announced that it’s COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601) had received Emergency Use…

On Oct. 2, 2023, France began vaccinating ducks against bird flu to try and stem the virus that…

On Oct. 2, 2023, the Nobel Prize in Physiology or medicine was awarded jointly to Katalin Kariko and…
On Oct. 2, 2023, the World Health Organization (WHO) announced it had recommended a new vaccine, R21/Matrix-M, developed…

On Sept. 29, 2023, The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced it…

On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 27, 2023, Gritstone bio announced that it was awarded a contract by the Biomedical Advanced Research…
On Sept. 20, 2023, the National Institutes of Health announced that a trial of a preventive HIV vaccine…

On Sept. 15, 2023, researchers at the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…
![European Commission expanded Merck’s ERVEBO [Ebola Zaire vaccine] indication to include children 1 year of age and older](https://www.lifesciencehistory.com/wp-content/uploads/2022/08/european_commission_logo-768x768.jpg)
On Sept. 7, 2023, Merck announced that the European Commission (EC) had approved an expanded indication for ERVEBO…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Aug. 25. 2023, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…
On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…
On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…

On Aug. 1, 2023, BD announced that the U.S. Food and Drug Administration (FDA) 510(k) had provided clearance…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
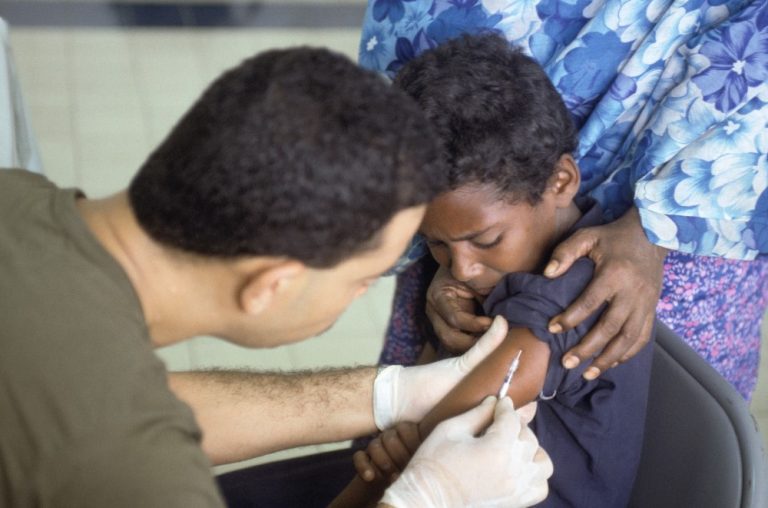
On Jul. 28, 2023, the World Health Organization (WHO) reported circulating vaccine-derived poliovirus type 2 (cVDPV2) in the…
On Jul. 28, 2023, COVID vaccine uptake among hospital workers at a Philadelphia teaching hospital rose from 86%…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…