JAMA reported excess death rates for Republican and Democratic voters in Florida and Ohio during COVID-19 pandemic
On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…

On Jul. 14, 2023, the UK Animal and Plant Health Agency reported that additional asymptomatic human detections of…

On Jul. 5, 2023, the IHR National Focal Point of Poland notified WHO of unusual deaths in cats…

On Jun. 26, 2023, the Centers for Disease Control and Prevention (CDC) announced they were collaborating with U.S….
On May 31, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On May 30, 2023, the United Kingdom of Great Britain and Northern Ireland reported to the World Health…

On May 26, 2023, Novavax announced that Nuvaxovid (NVX-CoV2373) had been recommended for full Marketing Authorization (MA) for…

On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…

On Apr. 27, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 14, 2023, the National Health Commission of the People’s Republic of China reported a confirmed case…
On Apr. 10, 2023, the U.S. Department of Health and Human Services (HHS) announced it was spending over…

On Apr. 5, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…
On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…
On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…
On Mar. 24, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Tuberculosis (TB) in…
On Mar. 20, 2023, NIH scientists announced that the magnitude and quality of a key immune cellメs response…
On Mar. 20, 2023, some of the world’s leading makers of flu vaccines said they could make hundreds…

On Mar. 17, 2023, the Indonesia Ministry of Health notified the World Health Organization (WHO) of the detection…
On Mar. 14, 2023, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) of the…
On Mar. 10, 2023, the U.S. Centers for Disease Control and Prevention (CDC) in a significant step toward…

On Mar. 6, 2023, Merck announced that the U.S. Food and Drug Administration had approved the addition of…

On Feb. 28, 2023, a team from the University of Sydney reported that a flu virus found in…

On Feb. 26, 2023, the Cambodia International Health Regulations (IHR) National Focal Point (NFP) reported one confirmed case…
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 24, 2023, Moderna announced will make certain contingent development, commercial and regulatory milestone payments to the…
On Feb. 22, 2023, the Centers for Disease Control and Prevention (CDC) reported that the 2022-2023 flu vaccines…

On Feb. 17, 2023, Moderna announced that Health Canada had authorized the use of its Omicron-targeting bivalent COVID-19…
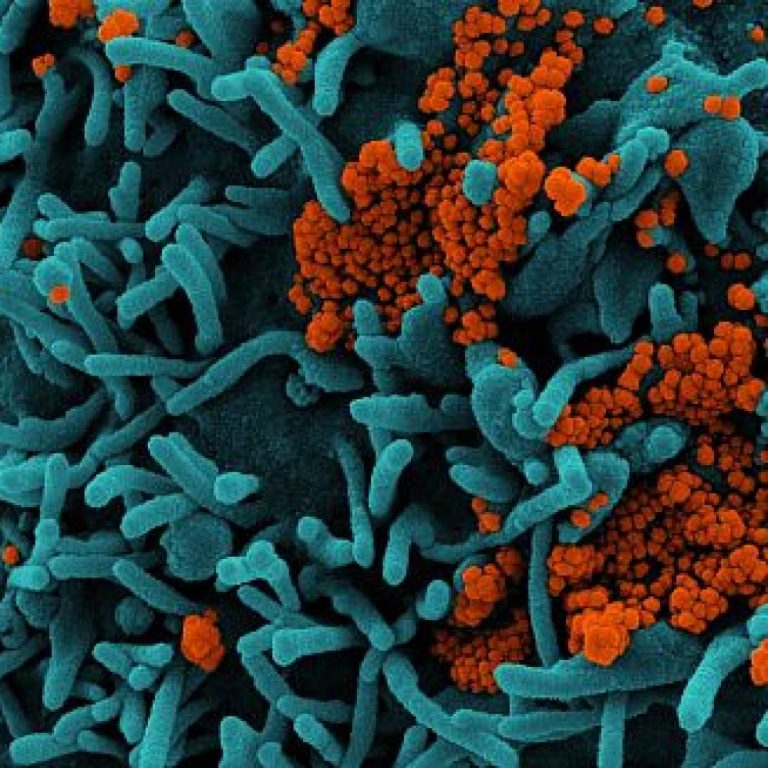
On Feb. 16, 2023, it was reported in the Lancet that protection from past infection against re-infection from…