Pfizer and BioNTech received positive CHMP ppinion for COMIRNATYᆴ in children 6 months to less than 5 lears in EU
On Oct. 19, 2022, Pfizer and BioNTech announced that the European Medicines Agencyメs (EMA) Committee for Medicinal Products…
On Oct. 19, 2022, Pfizer and BioNTech announced that the European Medicines Agencyメs (EMA) Committee for Medicinal Products…

On Oct. 18, 2022, global leaders confirmed US$ 2.6 billion in funding toward the Global Polio Eradication Initiative’s…

On Oct. 17, 2022, Moderna and Gavi, the Vaccine Alliance, announced they had mutually agreed to cancel remaining…
On Oct. 13, 2022, Novavax announced positive results from the Phase 1/2 clinical trial of its COVID-19-Influenza Combination…

On Oct. 13, 2022, Pfizer and BioNTech announced early data from a Phase 2/3 clinical trial (NCT05472038) evaluating…

On Oct. 13, 2022, researchers from the University of Oxford reported findings from a study exploring how certain…
On Oct. 12, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Oct. 11, 2022, researchers from the University of Oxford have reported new findings from a Phase 1…

On Oct. 10, 2022, Novavax announced that partner, SK bioscience, had submitted a Post Approval Change Application to…
On Oct. 10, 2022, Novavax announced that Switzerland’s Federal Office of Public Health had recommended Nuvaxovid (NVX-CoV2373) as…

On Oct. 7, 2022, the U.S. Food and Drug Administration approved Boostrix (Tetanus Toxoid, Reduced Diphtheria Toxoid and…

On Oct. 7, 2022, Pfizer and BioNTech announced that Health Canada had authorized COMIRNATY Original & Omicron BA.4/BA.5…

On Oct. 6, 2022, researchers in Japan released a study that described the development and application of the…
On Oct. 4, 2022, Washington University in St. Louis announced it had licensed the rights to develop, manufacture…
On Sept. 28, 2022, Pfizer and BioNTech announced they had completed a submission to the European Medicines Agency…

On Sept. 28, 2022, Moderna announced that the European Medicines Agency (EMA) had accepted a variation for the…

On Sept. 28, 2022, the Oregon Department of Agriculture and the U.S. Department of Agriculture’s Animal Plant Health…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced that a large international study had confirmed…

On Sept. 27, 2022, Novavax announced that an initial one million doses of Nuvaxovid (NVX-CoV2373) COVID-19 vaccine were…

On Sept. 16, 2022, Novavax announced that the Taiwan Food and Drug Administration had granted expanded emergency use…

On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…

On Sept. 15, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
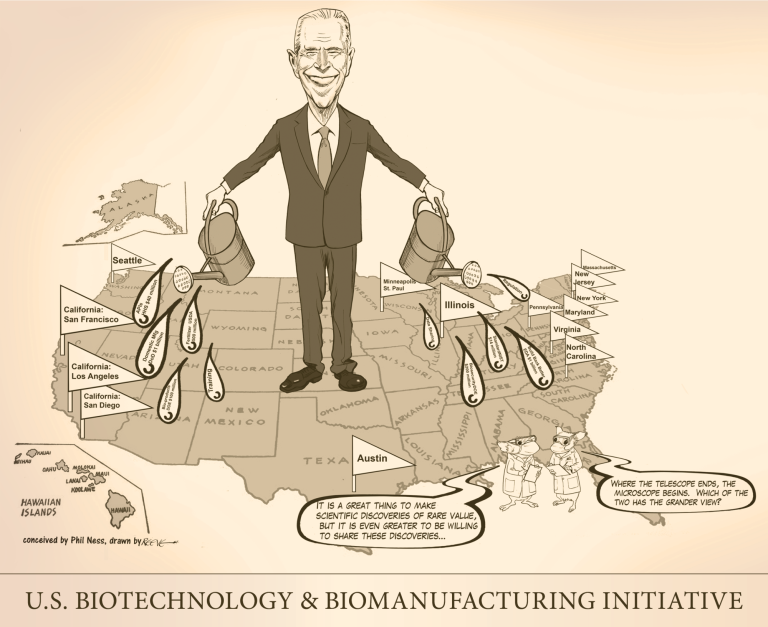
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…
On Sept. 13, 2022, Novavax and Serum Institute of India, the world’s largest vaccine manufacturer by volume, announced…

On Sept. 8, 2022, researchers from the University of Oxford and their partners reported findings from their Phase…

On Sept. 2, 2022, Novavax announced that Swissmedic, the Swiss Agency for Therapeutic Products, had expanded its temporary…

On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…

On Sept. 1, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional marketing…

On Aug. 29, 2022, the U.S. Department of Health and Human Services (HHS) announced it had provided approximately…

On Aug. 26, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…