Adjuvant developed with NIH funding enhanced efficacy of Indiaメs COVID-19 vaccine
On Jun. 29, 2021, the National Institutes of Health announced that an adjuvant developed with its funding had…

On Jun. 29, 2021, the National Institutes of Health announced that an adjuvant developed with its funding had…

On Jun. 29, 2021, Paratek Pharmaceuticals announced it had delivered the first procurement of NUZYRA to the Biomedical…
On Jun. 29, 2021, Moderna announced new results from in vitro neutralization studies of sera from individuals vaccinated…
On Jun. 29, 2021, Moderna announced that the government of India had issued a registration certificate and a…

On Jun. 29, 2021, Altimmune announced phase 1 AdCOVID clinical trial data that showed AdCOVID appeared to be…

On Jun. 29, 2021, VBI Vaccines announced positive Phase 1 data from its Phase 1/2 trial of the…

On Jun. 28, 2021, the University of Oxford in partnership with AstraZeneca began vaccinations for a new phase…

On Jun. 28, 2021, researchers running the University of Oxford-led Com-COV study reported that alternating doses of the…
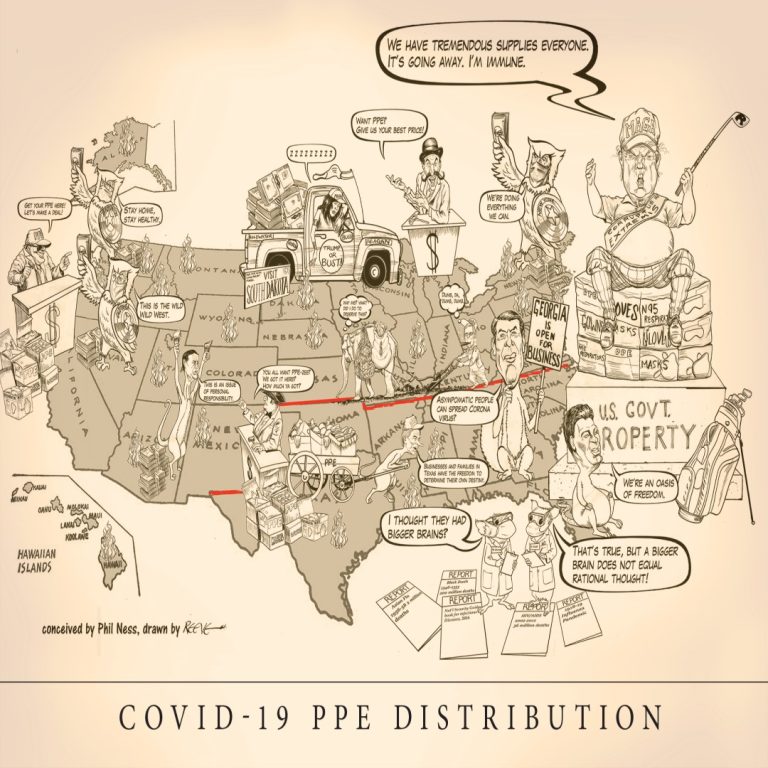
Cast of Characters: Jay Inslee, Governor, Washington | Gavin Newsom, Governor, California | Doug Ducey, Governor, Arizona |…

On Jun. 24, 2021, researchers from the University of Oxford released their findings about the so-called ムcorrelates of…

On Jun. 23, 2021, the National Institutes of Health announced that an observational study had begun to evaluate…
On Jun. 22, 2021, Moderna announced that the European Commission (EC) had purchased an additional 150 million doses…
On Jun. 22, 2021, the National Research Council of Canada (NRC) announced it had completed construction of the…

On Jun. 21, 2021, Tonix Pharmaceuticals announced that it planned to develop TNX-102 SL (cyclobenzaprine HCl sublingual tablets)…

On Oct. 6, 2021, the World Health Organization and its COVAX partners are working with a South African…
On Jun. 17, 2021, the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research granted…

On Jun. 16, 2021, CureVac announced results of the second interim analysis of its international pivotal Phase 2b/3…

On Jun. 16, 2021, Moderna announced that the U.S. government had purchased an additional 200 million doses of…

On Jun. 16, 2021, Regeneron announced positive results from the largest trial assessing any monoclonal antibody treatment in…

On Jun. 16, 2021, Pfizer and The Academic Research Organization (ARO) from the Hospital Israelita Albert Einstein announced…

On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…

On Jun. 15, 2021, Moderna and Magenta Investments, an industrial conglomerate in the United Arab Emirates (UAE), announced…

On Jun. 14, 2021, University of British Columbia researchers Dr. Jayachandran Kizhakkedathu, Dr. Caigan Du and Dr. Christopher…

On Jun. 14, 2021, Moderna announced that it had submitted an authorization application to Swissmedic for use of…

On Jun. 14, 2021, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, demonstrated 100% protection against…
On Jun. 14, 2021, Novavax announced data from the first co-administration study of a SARS-CoV-2 vaccine candidate [Novavax,…

On Jun. 13, 2021, G7 global leaders pledged to share COVID-19 vaccine doses internationally, in support of global…
On Jun. 11, 2021, Moderna and Tabuk Pharmaceutical announced an agreement to commercialize the Moderna COVID-19 Vaccine and…
On Jun. 11, 2021, Novavax announced preclinical and clinical data on the company’s original recombinant protein COVID-19 vaccine…

On Jun. 10, 2021, Moderna announced that it had requested an emergency use authorization (EUA) for its COVID-19…