The U.S. Surgeon General issued landmark Report on Alcohol, Drugs, and Health
On Nov. 17, 2016, the U.S. Surgeon General Dr. Vivek Murthy issued a landmark Report on Alcohol, Drugs,…

On Nov. 17, 2016, the U.S. Surgeon General Dr. Vivek Murthy issued a landmark Report on Alcohol, Drugs,…

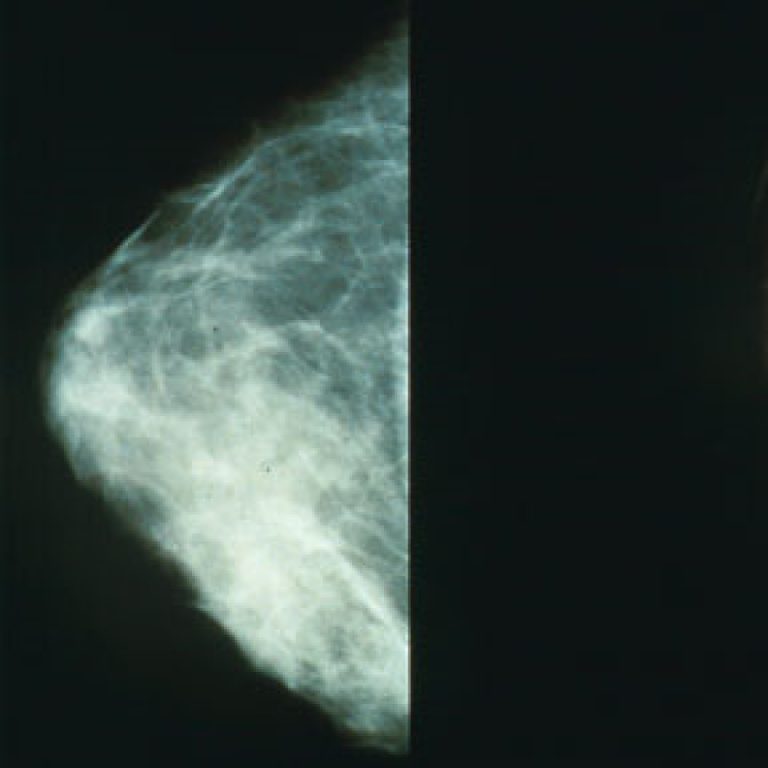
On Jan. 11, 2016, the U.S. Preventive Services Task Force (USPSTF) recommended biennial screening mammography for women aged…

On Dec, 22, 2015, the International Day of Women and Girls in Science was established by resolution of…

On Jun. 16, 2015, Ardis Dee Hoven became the first woman president of the World Medical Association (WMA)….
On Apr. 29, 2015, the Pan American Health Organization ᅠdeclared that the Americas region has become the first…

On Mar. 2, 2015, Dr. Helen Wallace, a world-renowned professor, mentor and advocate known for her passion for…

On Nov. 21, 2014, funding was announced for the TEC Health Accelerator for Alberta, to assist promising startup…
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…

On Apr. 14, 2014, Stanford Medicine researchers announced a study that found a gene variant puts women at…

On May 8, 2013, was the first World Ovarian Cancer Day. On this day, ovarian cancer organizations from…
On Feb. 22, 2013, the Advisory Committee on Immunization Practices (ACIP) recommended that unvaccinated pregnant women receive a…

On Oct. 24, 2012, the Immunization Practices Advisory Committee (ACIP) voted 14-0 with one abstention to recommend that…
On Jun. 8, 2012, the U.S. Food and Drug Administration (FDA) announced that Genentech’s drug Perjeta (pertuzumab) was…

On Apr. 30, 2012, Hologic announced the acquisition of San Diego-based Gen-Probe, a molecular diagnostics company, for $3.7…

On Apr. 30, 2011, The University of Minnesota Masonic Children’s Hospital opened a new, innovative building for mothers…
On Mar. 10, 2010, National Women and Girls HIV/AIDS Awareness Day (NWGHAAD) was established as a nationwide initiative…

On Mar. 5, 2010, the National Cancer Institute (NCI) announced that it had awarded the Siteman Cancer Center…

On Oct. 16, 2009, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Cervarix [Human papillomavirus…

In 2008, Evestra was spun-off as a private biopharmaceutical company from the Texas Biomed Department of Organic Chemistry….

In 2008, the Seattle Cancer Care Alliance introduced the Mammovan, making digital mammography more accessible to women throughout…

On Dec. 12, 2007, the Breast Cancer Research Stamp Reauthorization Act extended through Dec. 31, 2011, provisions requiring…
On Nov. 27, 2007, a new National Cancer Institute (NCI) model for calculating invasive breast cancer risk, called…

On Sept. 14, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved Eli Lilly’s osteoporosis…

On Apr. 20, 2007, the National Breast and Cervical Cancer Early Detection Program Reauthorization Act of 2007 allowed…

On Apr. 18, 2007, a study led by the University of Texas M.D. Anderson Cancer Center reported that…

On Mar. 28, 2007, Magnetic Resonance Imaging (MRI) scans of women who were diagnosed with cancer in one…
On Nov. 16, 2006, the U.S. Food and Drug Administration (FDA) approved Genentech’s trastuzumab (Herceptin) for use with…

On May 23, 2006, the Trial Assigning IndividuaLized Options for Treatment (Rx), or TAILORx, was launched to examine…
On Apr. 17, 2006, the National Cancer Institute (NCI) announced initial results of the Study of Tamoxifen and…

In 2006, the National Cancer Institute (NCI) launched the TAILORx trial to determine whether gene expression patterns in…