Moderna announced supply agreement with U.S. Government for initial 100 million doses of mRNA vaccine against COVID-19
On Aug. 11, 2020, Moderna announced that the U.S. government had secured 100 million doses of mRNA-1273. The…

On Aug. 11, 2020, Moderna announced that the U.S. government had secured 100 million doses of mRNA-1273. The…
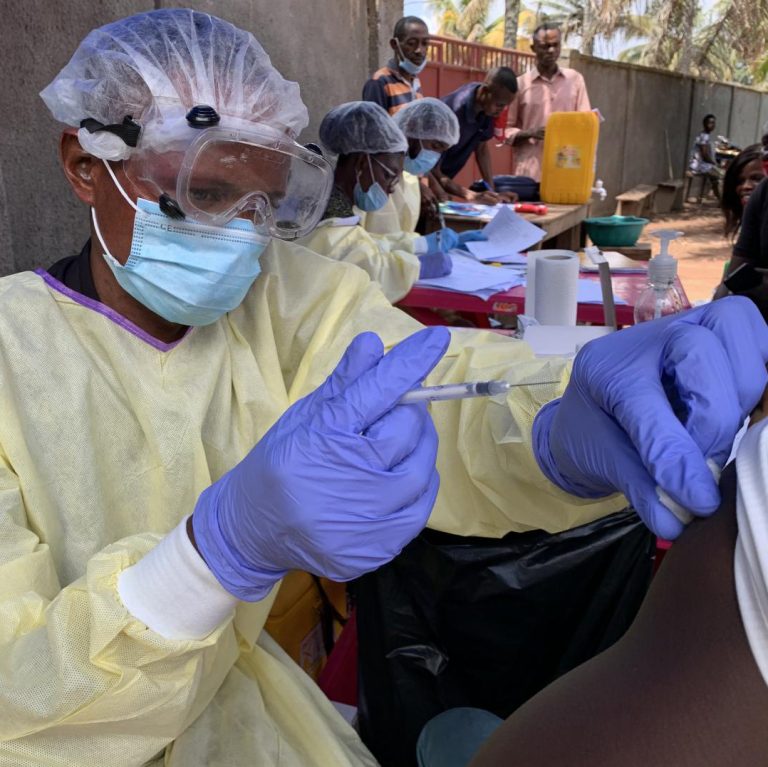
On Jul. 29, 2020, Regeneron announced that the Biomedical Advanced Research and Development Authority (BARDA) had entered into…

On Jul. 26, 2020, Moderna announced a modification to its contract with the Biomedical Advanced Research and Development…
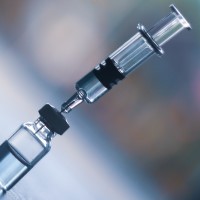
On Jul. 8, 2020, BD (Becton, Dickinson) announced the formation of a strategic, public-private partnership with the Biomedical…

On Jul. 7, 2020, Regeneron announced that, as part of Operation Warp Speed, the Biomedical Advanced Research and…

On Jun. 30, 2020, Altimmune announced dosing of the first patient in the Company’s Phase 1b clinical trial…
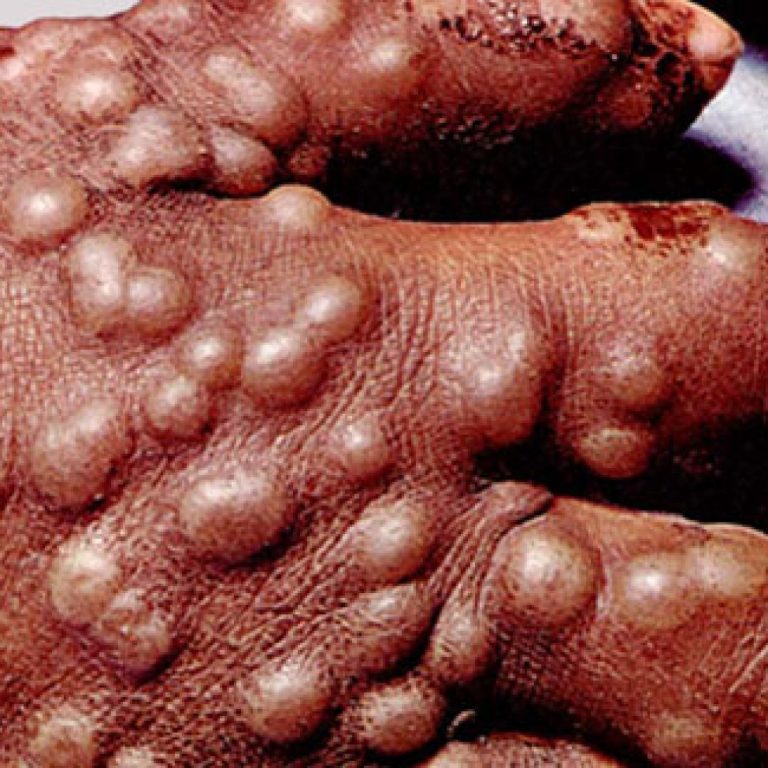
On Jun. 25, 2020, SIGA Technologies announced the deliveries of oral TPOXX (tecovirimat) to the U.S. Department of…

On Jun. 11, 2020, Quidel announced it had received funding from the Biomedical Advanced Research and Development Authority…

On Jun. 10, 2020, OraSure Technologies announced it had been awarded a $629,217 contract from the Biomedical Advanced…

On Jun. 1, 2020, Emergent BioSolutions announced it had been issued a task order under an existing contract…

On May 19, 2020, Phlow, a U.S.-based, public benefit drug manufacturing corporation, received federal government funding of $354…

On May 4, 2020, Mayo Clinic was awarded a $26 million contract from the Biomedical Advanced Research and…
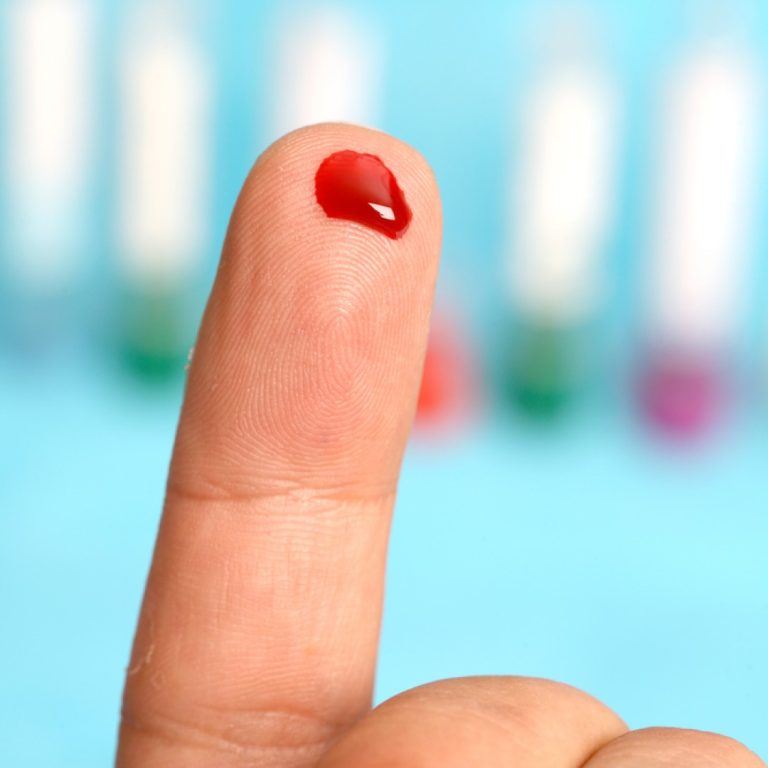
On Apr. 30, 2020, InBios announced that it had been awarded a contract from the Biomedical Advanced Research…
On Apr. 27, 2020, Moderna announced it had submitted an Investigational New Drug application to the U.S. Food…

On Apr. 22, 2020, the Biomedical Advanced Research and Development Authority (BARDA) and Tangen Biosciences announced teaming up…
On Apr. 16, 2020, Moderna announced an agreement for a commitment of up to $483 million from the…
On Apr. 8, 2020, Nanomix announced that the company had been awarded $570,000 in funding from Biomedical Advanced…

On Apr. 6, 2020, OraSure Technologies announced it had been awarded a $710,310 contract from the Biomedical Advanced…
On Apr. 2, 2020, Emergent BioSolutions announced it had entered into a formal partnership with the U.S. government…

On Apr. 1, 2020, Innovation Pharma announced BARDA had initiated funding to support the onshoring of Paratek manufacturing…

On Mar. 31, 2020, Cue Health announced that is had been awarded up to $30 million in base…

On Mar. 31, 2020, Philips announced that it was fully committed to fulfill the contract with the U.S….
On Mar. 30, 2020, Altimmune announced that it was launching a collaboration with the University of Alabama at…

On Mar. 19, 2020, Mesa Biotech announced it had been awarded a $561,000 contract from the U.S. Department…
On Mar. 19, 2020, Roche announced it was working with the U.S. Food & Drug Administration (FDA) to…
On Feb. 18, 2020, Sanofi announced it had leveraged previous development work for a SARS vaccine which may…

On Jul. 12, 2018, Cue Health announced that is had been awarded up to $30 million in base…
On Jul. 13, 2010, Bavarian Nordic announced that it had delivered 1 million doses of its smallpox vaccine…