Femasys Secures Regulatory Approval for FemBloc® Permanent Birth Control in New Zealand
On Sept. 8, 2025, Femasys announced it has received approval from the New Zealand Medicines and Medical Devices…

On Sept. 8, 2025, Femasys announced it has received approval from the New Zealand Medicines and Medical Devices…

On Jun. 25, 2025, Femasys announces Conformité Européene (CE) mark certification under European Union Medical Device Regulation of…
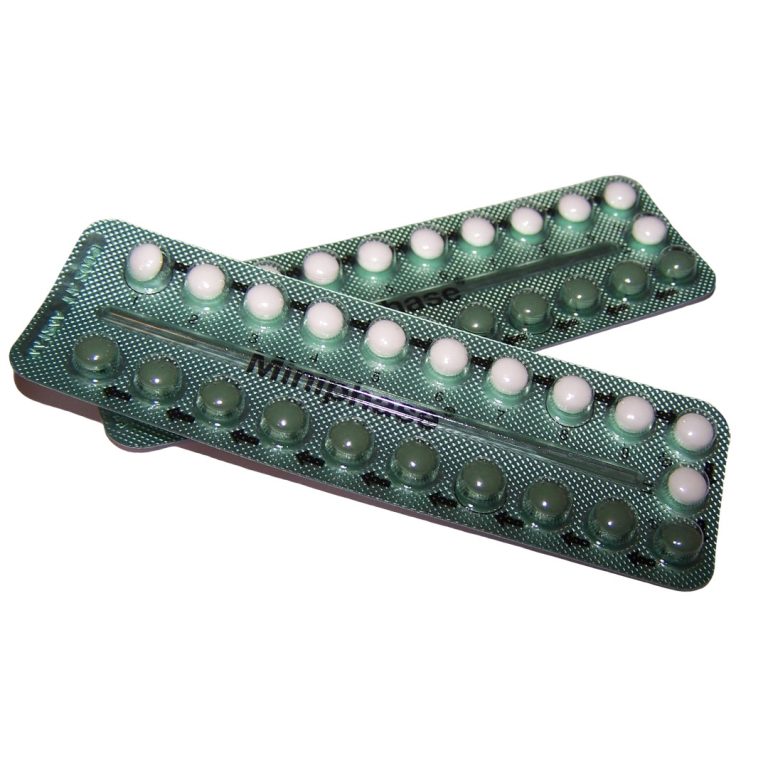
In 1970, the U.S. Food and Drug Administration (FDA) required the first patient package insert: oral contraceptives must contain…