Abbott announced discovery of a new strain of HIV
On Nov. 6, 2019, Abbott announced that a team of its scientists identified a new subtype of the…
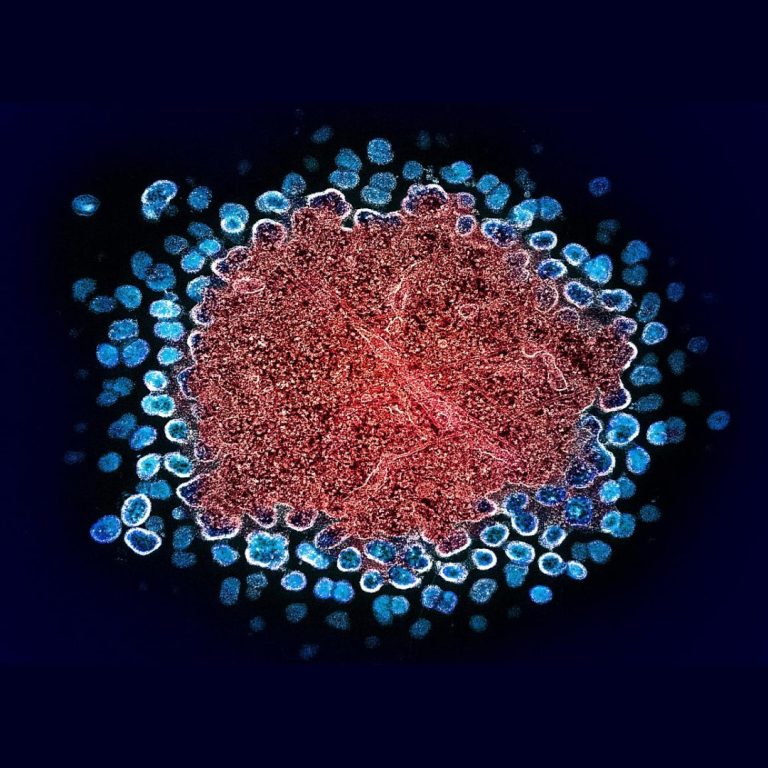
On Nov. 6, 2019, Abbott announced that a team of its scientists identified a new subtype of the…
On Feb. 15, 2019, the U.S. Centers for Disease Control and Prevention (CDC) published ACIP recommendations for use….
On Dec. 26, 2018, Sanofi announced the U.S. Food and Drug Administration (FDA) had approved VAXELIS (Diphtheria and…

On Nov. 2, 2018, the Immunization Practices Advisory Committee (ACIP) published updated recommendations on use of hepatitis A…
On Apr. 20, 2018, the Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee (ACIP)…

On Jan. 12, 2018, the U.S. Centers for Disease Control and Prevention (CDC) published updated Immunization Practices Advisory…

On Nov. 9, 2017, Dynavax Technologies announced that the U.S. Food and Drug Administration (FDA) had approved HEPLISAV-B…

On Sept. 15, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated GamaSTAN S/D prescribing…

On Aug. 28, 2017, the American Academy of Pediatrics (AAP) issued policy stating that newborns should routinely receive…
On Feb. 10, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published the 2017 U.S. recommended…

On Sept. 13, 2016, Arbutus Biopharma announced that Dr. Michael J. Sofia, Arbutus’ Chief Scientific Officer, had been…

On Dec. 20, 2013, the U.S. Centers for Disease Control and Prevention (CDC) published guidance for HBV protection…

On Feb. 25, 2009, the Advisory Committee on Immunization Practices (ACIP) recommended routine hepatitis A vaccination for household…

On Oct. 27, 2008, the National Quality Forum included the hepatitis B birth dose among its consensus standards…
On Oct. 19, 2007, the U.S. Centers for Disease Control and Prevention (CDC) published updated recommendations for prevention…
On May 8, 2007, the National Cancer Institute (NCI) reported that people infected with the hepatitis C virus…
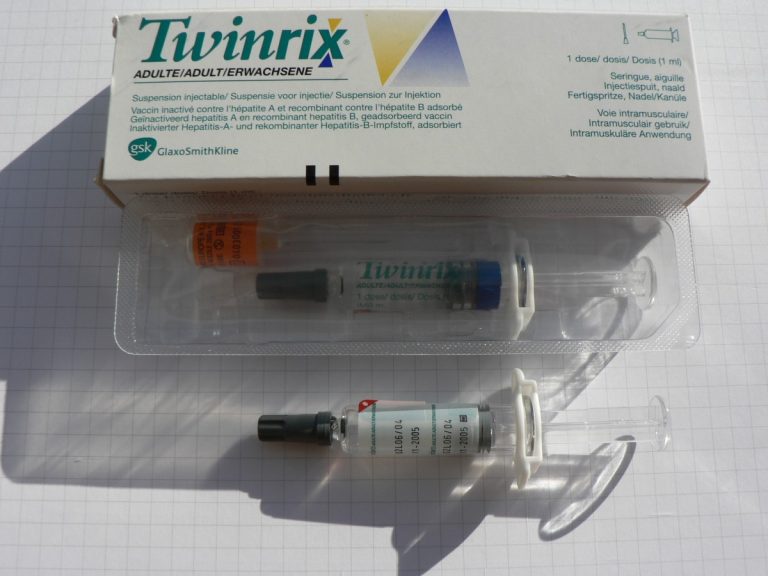
On Mar. 28, 2007, the U.S. Food and Drug Administration (FDA) announced that GlaxoSmithKline (King of Prussia, Pennsylvania)…
On Dec. 23, 2005, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
On Oct. 17, 2005, the FDA approved lowering the age limit to 12 mos for the remaining U.S.-licensed…

On Aug. 11, 2005, the Food and Drug Administration (FDA) approved an application of a pediatric/adolescent formulation of…

On Dec. 13, 2002, the U.S. Food and Drug Administration (FDA) announced it had licensed a combined diphtheria…

On Dec. 3, 2002, Genentech drug Pegasys (peginterferon alfa-2a) was approved by the Food and Drug Administration (FDA)…

In 2002, Stanford geneticist Mark Kay uses a gene-therapy technique known as RNA inhibition to switch off genes…

On May 11, 2001, the Food and Drug Administration (FDA) licensed a combined hepatitis A and B vaccine…

On Aug. 29, 1999, Merck, West Point, Pennsylvania) received approval from the FDA of a supplement to Merck’s…

On Jan. 15. 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
On Aug. 26, 1998, Bill and Melinda Gates announced the establishment of the Bill and Melinda Gates Children’s Vaccine…

In 1998, some research caused concern that hepatitis B vaccination might be linked with multiple sclerosis (MS), a…

On Oct. 2, 1996, the Food and Drug Administration (FDA) licensed a combined Hib conjugate and hepatitis B…

On Mar. 29, 1996, the second inactivated hepatitis A vaccine (Vaqta by Merck) was licensed.